1816
Upregulation of hippocampal glutamatergic neurotransmission during acute episodes of major depression: Excitotoxic effects might be related to reduced hippocampal volumes1Department of Clinical Radiology, University of Muenster, Muenster, Germany, 2Department of Psychiatry, University of Muenster, Muenster, Germany
Synopsis
Investigation of the glutamatergic metabolism with 1H-spectroscopy revealed a significant higher glutamate level in the hippocampus in patients with major depression. The excitotoxicity of increased glutamate levels on neural brain structures might be causally related to reduced volumes of hippocampi as found in patients with recurrend episodes.
INTRODUCTION
Major depressive disorder (MDD) is a common and recurrent illness affecting more than 120 million people worldwide.1 Antidepressant medication increases the synaptic availability of serotonin, norepinephrine or dopamine in the synaptic cleft and thus supports the monoamine hypothesis of depression. Still, there has been increasing evidence that the glutamatergic system is involved in the pathophysiology of MDD and recent evidence suggests that directly altering glutamatergic neurotransmission with medications such as ketamine may produce much more rapid and robust antidepressant effects.2 Studies investigating glutamate in blood and CSF reported increased levels of glutamate in MDD patients. Studies with proton magnetic resonance spectroscopy (1H-MRS) have mostly investigated prefrontal brain areas with reduced glutamate and Glx levels especially in the ACC.3 Reduced hippo-campus volumes have been found in multiple psychiatric disorders including major depression4, and the hippocampus is a plastic and very vulnerable brain region most sensitive to stress.5 We used 1H-MRS to investigate glutamate in the hippocampus in acutely depressed patients and hypothesized increased levels of glutamate in the patient group.METHODS
38 patients (mean age 43,0 ± 10,7 years), meeting DSM-IV criteria (SKID I) for major depressive disorder and 40 age matched healthy controls (mean age 38,7 ± 10,6 years), were finally enrolled in our study. Written informed consent was obtained after oral explanation of the study procedures. Exclusion criteria were lifetime diagnosis of schizophrenia or bipolar I disorder, current drug or alcohol abuse, severe medical disorders, recent or acute head injury and any exclusion criteria for MRI. Participants underwent the following psychometric assessments: Ruminative-Response-Scale RRS6, Perseverative Thinking Questionnaire PTQ7, the Becks Depression Inventory BDI-II8.
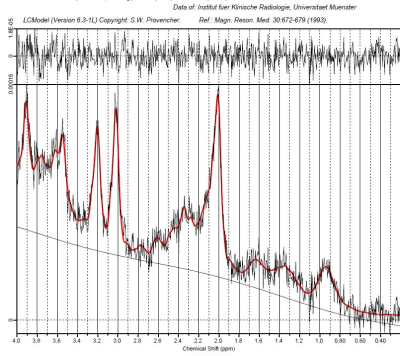
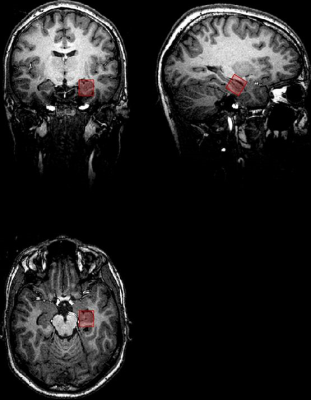
Single Voxel 1H magnetic resonance spectroscopy was performed on a 3T scanner (SIEMENS PRISMA fit) using a Point Resolved Spectroscopy Sequence (PRESS) with the following parameters: TE 32 ms, TR 2000 ms, 2048 datapoints, bandwidth 1200 Hz, an iterative shim procedure and water suppression. Quantification of the spectra was based on LCModel spectral fitting.9 The volume of interest (VOI) was placed in the anterior part of the left hippocampus. Additionally a high resolution T1 weighted 3D dataset was acquired. All concentration values were normalized to the unsuppressed water signal from the same VOI and expressed in institutional units (IU). The following metabolites were quantified: N-acetyl-aspartate with N-acetyl-aspartyl-glutamate (tNAA), glycerophosphocholine with phosphocholine (tCh), glutamate (Glu), and glutamine and glutamate (Glx). Only metabolite concentrations with Cramér-Rao lower bounds (CRLBs) below 20% were accepted and used for the following analyses.
RESULTS
Patients with major depression scored significantly higher in all measures of depression. In the hippocampus, glutamate (p=0.002) and Glx (p=0.031) levels were significantly higher in patients than controls, all other metabolites revealed no differences. There was no correlation between clinical data and glutamate or Glx levels, also there were no differences between male and female patients.DISCUSSION & CONCLUSION
Our results support the idea of an upregulated glutamatergic neurotransmission in the hippocampus. So far, in-vivo data of glutamate in the hippocampus in MDD has been sparse, Block et al10 reported reduced metabolite ratios of glutamine and Glx in the hippocampus, whereas a large study in MDD patients treated with ECT also found increased levels of Glx in the hippocampus which normalized under ECT.11 As the excitotoxicity of increased glutamate levels on neural brain structures is regarded a major pathomechanism for the effects of acute stress, glutamatergic dysregulation in MDD patients might be causally related to reduced volumes of hippocampi. Also, smaller hippocampi have been found in patients with recurrent episodes, not in first episode MDD patients, supporting the hypothesis of chronicity and number of episodes related pathology which might be normalized by antidepressant medication. Interestingly, Haroon et al12 hypothesized that in MDD the effects of inflammation on glia initially lead to an increased release of glutamate into the extrasynaptic space by decreasing the capacity of glial transporters to clear glutamate from the synaptic cleft. A very recent study in patient with posttraumatic stress disorder which is also related with smaller hippocampi found significantly increased levels of glutamate, thus supporting the hypothesis of stress related excitotoxic effects of elevated glutamate levels on the hippocampus.Acknowledgements
No acknowledgement found.References
1. Belmaker, RH, Agam G. Major depressive disorder. N Engl J Med 2008;358: 55-68.
2. Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression. Neuropsychopharmacology 2012;62(1):63-77.
3. Luykx JJ, Laban KG, van den Heuvel MP, Boks MP, Mandl RC, Kahn RS, Bakker SC. Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of (1)H-MRS findings. Neurosci Biobehav Rev. 2012;36(1):198-205.
4. Schmaal L, Veltman DJ, van Erp TGM, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 2016;21(6):806-812.
5. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007 Jul;87(3):873-904.
6. Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination Reconsidered: A Psychometric Analysis. Cognitive Therapy and Research; June 2003,27(3):247–25.
7. Ehring T, Zetsche U, Weidacker K, Wahl K, Schönefeld S, Ehlers A. The Perseverative Thinking Questionnaire (PTQ): validation of a content-independent measure of repetitive negative thinking. J Behav Ther Exp Psychiatry 2011;42(2):225-32.
8. Beck A, Steer R, & Brown G. Beck Depression Inventory-Second Edition (BDI-II). The Psychological Corporation, 555 Academic Court, San Antonio, TX 1996.
9. Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 30(6):672- 679.
10. Block W, Träber F, von Widdern O, Metten M, Schild H, Maier W, Zobel A, Jessen F. Proton MR spectroscopy of the hippocampus at 3 T in patients with unipolar major depressive disorder: correlates and predictors of treatment response. Int J Neuropsychopharmacol. 2009;12(3):415-22.
11. Njau S, Joshi SH, Espinoza R, Leaver AM, Vasavada M, Marquina A, Woods RP, Narr KL. Neurochemical correlates of rapid treatment response to electroconvulsive therapy in patients with major depression. J Psychiatry Neurosci. 2017;42(1):6-16.
12. Haroon E, Miller AH, Sanacora G. Inflammation, glutamate, and glia: a trio of trouble in mood disorders. Neuropsychopharmacology. 2017;42:193-215.