1810
Atypical associations between language comprehension network and attention pathways in autism spectrum disorders1Institute of Medical Device and Imaging, National Taiwan University College of Medicine, Taipei, Taiwan, 2The Ph.D. Program for Neural Regenerative Medicine, Taipei Medical University, Taipei, Taiwan, 3Department of Psychiatry, National Taiwan University Hospital and College of Medicine, Taipei, Taiwan
Synopsis
Impaired language comprehension has been consistently found in autism spectrum disorder (ASD). Development of language comprehension highly corresponds to joint attention and impulsivity. We used diffusion spectrum imaging to measure white matter integrity of the language comprehension network and the attention pathways in 60 ASD and 55 typically developing (TD) boys. ASD showed partially reduced white matter integrity in the targeted tracts as compared to TD. The tract covariance between the language comprehension network and the attention pathways showed different patterns in both groups which may shed light in the relationships of language and attention in ASD.
Introduction
Autism spectrum disorders (ASD) show varied severities of deficits in language comprehension. Individuals with ASD showed worse performance than typically developing (TD) people in pragmatic skills 1 and complex language tasks such as comprehension and inference 2, 3. Moreover, attention impairment is considered to be one of the key cognitive deficits leading to communication deficits in ASD 4, 5. Recently, an evolving cortical network underlying language comprehension was reported 6. The language comprehension network in relation to developmental trajectories includes the left uncinate fasciculus (UF) for phrase structure reconstruction, right UF for prosodic processing, left ventral pathway for linguistic processing, right ventral pathway for prosodic processing, and left dorsal pathway for semantic/syntactic relation 6 which corresponded to the dual stream model for language proposed by Saur and colleagues 7. The attention pathways responsible for impulsivity include bilateral frontal-striatal (FS) tracts 8 and bilateral anterior cingulum 4. Given that the language comprehension network and the attention pathways correspond to deficits in communication and impulsivity 9 in ASD, we hypothesized that the correlations between the language comprehension network and the attention pathways would show different patterns in both groups.Methods
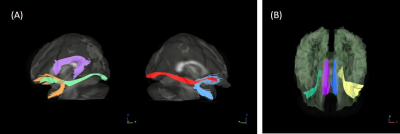
Sixty right-handed boys with ASD and 55 TD boys (aged 10 to 18) received clinical evaluations and MRI scans. Diffusion spectrum imaging (DSI) was used to measure the microstructural integrity of the targeted white matter tracts in the study. All DSI data were acquired using a twice-refocused balanced echo diffusion echo planar imaging sequence. 102 diffusion encoding gradients with the maximum diffusion sensitivity bmax = 4000 s/mm2 were sampled on the grid points in a half sphere of the 3D q-space. A template-based approach was employed to measure the targeted tract integrity 10. Targeted fiber tract bundles were segmented in a DSI template (NTU-DSI-122) 11 (Figure 1), in which multiple regions of interest (ROIs) were selected for each targeted tract bundle by using an automated anatomical labeling system. After the ROIs for each targeted tract bundle were selected, streamline-based fiber tracking was performed based on the resolved fiber vector fields provided by DSI in the NTU-DSI-122 template. DSI tractography was performed using in-house software (DSI Studio: http://dsi-studio.labsolver.org). A template-based approach was implemented in which the coordinates of the segmented tract bundles were transformed from the NTU-DSI-122 template to the study-specific template (SST), and from the SST to individual DSI datasets to sample the tract integrity indices along each of the tracts. The tract integrity of each targeted tract bundle was determined by averaging the general fractional anisotropy (GFA) values along each tract bundle. The tract covariance was calculated by computing the correlations between mean GFA of a tract bundle in the language comprehension network and that in the attention pathways. The tract covariance indicates the relatedness of a tract with another tract.Results
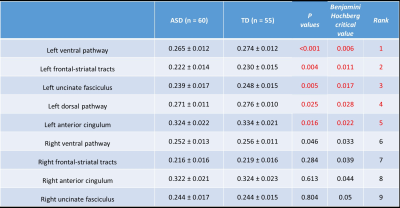
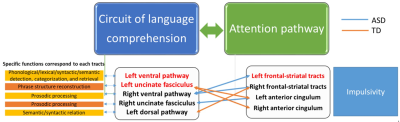
Applying Benjamini-Hochberg method for multiple testing, boys with ASD had significantly lower averaged GFA in the left dorsal pathway, bilateral ventral pathways, left UF, left anterior cingulum, and left FS tracts than did the TD boys (Figure 2). After partial correlation controlling for age factor, significant correlations were found between the left UF and bilateral anterior cingulum (left, p = 0.003; right, p = 0.003); the left dorsal pathway and right FS tracts (p = 0.001) in TD. In ASD, there was correlations between the right ventral pathway and bilateral FS tracts (left, p < 0.001; right, p < 0.001); as well as between the right UF and right anterior cingulum (p = 0.003) (Figure 3).Discussion
The present DSI study investigated the alteration and associations of the white matter integrity of the language comprehension network with the attention pathways in ASD and TD. The findings imply that the microstructural integrity of the language comprehension network and the attention pathways may attribute to the biological basis of deficits in language comprehension in ASD. Regarding the tract covariance, prosodic processing might be highly associated with impulsivity in ASD. However, lexical-semantic /syntactic functions might be correlated with impulsivity in TD (Figure 3). Therefore, the distinct patterns of the correlations between the language comprehension network and the attention pathways may shed light in the relationships of language comprehension and impulsivity in ASD.Conclusion
The findings support our hypothesis that an altered microstructural integrity and atypical tract covariance patterns of the language comprehension network and the attention pathways might be a structural correlate of deficits in language and attention in ASD.Acknowledgements
No acknowledgement found.References
1. Knaus TA, Silver AM, Lindgren KA, Hadjikhani N, Tager-Flusberg H. Fmri activation during a language task in adolescents with asd. J Int Neuropsychol Soc. 2008;14:967-979
2. Ames CS, Jarrold C. The problem with using eye-gaze to infer desire: A deficit of cue inference in children with autism spectrum disorder? J Autism Dev Disord. 2007;37:1761-1775
3. Stockbridge MD, Happe FG, White SJ. Impaired comprehension of alternating syntactic constructions in autism. Autism Res. 2013
4. Keehn B, Muller RA, Townsend J. Atypical attentional networks and the emergence of autism. Neurosci Biobehav Rev. 2013;37:164-183
5. Schietecatte I, Roeyers H, Warreyn P. Exploring the nature of joint attention impairments in young children with autism spectrum disorder: Associated social and cognitive skills. J Autism Dev Disord. 2012;42:1-12
6. Skeide MA, Friederici AD. The ontogeny of the cortical language network. Nat Rev Neurosci. 2016;17:323-332
7. Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, Umarova R, Musso M, Glauche V, Abel S, Huber W, Rijntjes M, Hennig J, Weiller C. Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A. 2008;105:18035-18040
8. Konrad A, Dielentheis TF, El Masri D, Bayerl M, Fehr C, Gesierich T, Vucurevic G, Stoeter P, Winterer G. Disturbed structural connectivity is related to inattention and impulsivity in adult attention deficit hyperactivity disorder. Eur J Neurosci. 2010;31:912-919
9. Langen M, Leemans A, Johnston P, Ecker C, Daly E, Murphy CM, Dell'acqua F, Durston S, Consortium A, Murphy DG. Fronto-striatal circuitry and inhibitory control in autism: Findings from diffusion tensor imaging tractography. Cortex; a journal devoted to the study of the nervous system and behavior. 2012;48:183-193
10. Chen YJ, Lo YC, Hsu YC, Fan CC, Hwang TJ, Liu CM, Chien YL, Hsieh MH, Liu CC, Hwu HG, Tseng WY. Automatic whole brain tract-based analysis using predefined tracts in a diffusion spectrum imaging template and an accurate registration strategy. Hum Brain Mapp. 2015;36:3441-3458
11. Hsu YC, Lo YC, Chen YJ, Wedeen VJ, Isaac Tseng WY. Ntu-dsi-122: A diffusion spectrum imaging template with high anatomical matching to the icbm-152 space. Hum Brain Mapp. 2015;36:3528-3541
Figures