1798
REDUCED INTRACRANIAL VOLUME IN FABRY DISEASE: A VOLUMETRIC MRI STUDYGiuseppe Pontillo1, Sirio Cocozza1, Arturo Brunetti1, Vincenzo Brescia Morra2, Eleonora Riccio2, Camilla Russo1, Francesco Saccà2, Enrico Tedeschi1, Antonio Pisani2, and Mario Quarantelli3
1Department of Advanced Medical Sciences, University of Naples Federico II, Naples, Italy, 2University of Naples Federico II, Naples, Italy, 3Institute of Biostructure and Bioimaging, National Research Council, Naples, Italy
Synopsis
To investigate the possibility that in Fabry Disease (FD), similarly to other LSD, an abnormal brain development could occur, we performed a volumetric MRI analysis on 42 FD patients and 38 healthy controls (HC). MRI data were processed using SPM12 to obtain ICV values, as well as brain parenchymal (BPF) and gray matter (GMF) fractions. Mean ICV of FD patients was 8.1% smaller compared to HC (p < 5·10-5), without significant differences in terms of BPF or GMF, thus suggesting a harmonious volumetric reduction of intracranial structures, as a reflection of a possible abnormal brain development in this condition.
Introduction
Fabry disease (FD) is a rare, X-linked disorder characterized by a progressive accumulation of globotriaosylceramide (Gb3) and related glycosphingolipids in different cells 1, 2. In contrast to many other lysosomal storage disorders (LSD) 3, 4, FD is regarded as a clinical adult-onset multisystemic condition2. Despite the frequency of brain involvement in FD, the physiopathology of CNS alterations in this condition has not yet been completely understood. Along with vascular and neurodegenerative mechanisms, both due to glycosphingolipid accumulation, the possibility that in FD an abnormal development of CNS could occur has never been fully investigated. The aim of our study was to perform a volumetric analysis of intracranial tissues in FD, investigating eventual differences in terms of total intracranial volume (ICV) between FD patients and a group of healthy controls (HC) as a possible expression of abnormal brain development in this condition.Methods
Forty-two patients with genetically proven FD were recruited, along with thirty-eight HC of comparable age and sex. All MRI exams have been carried out on the same 3 Tesla MR scanner and included an isotropic T1w acquisition for the volumetric analysis5, which was conducted using the Statistical Parametric Mapping (SPM12) software package (http://www.fil.ion.ucl.ac.uk/spm). For ICV and global GM, WM and cerebrospinal fluid (CSF) volumes estimation, structural data were processed using the unified segmentation tool 6; then, ICV was computed with the “tissue volumes” utility by adding up the segmented GM, WM and CSF volumes 7. To investigate possible changes in brain or GM volumes independent from ICV, brain parenchymal volume was defined as the sum of GM and WM, and normalized volumes were calculated as the ratio to ICV, thus obtaining brain parenchymal fraction (BPF) and gray matter fraction (GMF), respectively 8, 9. Finally, as a post-hoc evaluation, a voxel based morphometry (VBM) analysis was carried out to investigate possible regional GM differences between the two groups, as described in previous works10, 11.Results
FD patients showed significantly smaller intracranial volumes compared to HC, with a mean ICV that was 8.1% lower compared to the control group (1267.8 ± 121.5 ml vs 1379.8 ± 137.2 ml in FD and HC, respectively; p < 5·10-5). No significant differences emerged between the two groups when comparing both the BPF (81.2 ± 4.4% vs 81.5 ± 4.0% in FD and HC, respectively; p = 0.86) and the GMF (50.5 ± 3.6% vs 50.1 ± 3.9% in FD and HC, respectively; p = 0.21), while the post-hoc VBM analysis revealed a cluster of reduced GM density in FD patients compared to HC at the level of the thalami, bilaterally, extending towards the left hippocampus (p = 0.001) (Figure 1). No significant clusters of increased GM density were found in FD patients compared to HC.Discussion
ICV is defined as the sum of GM, WM and CSF, and it is a representation of the maximal brain growth obtained during development and maturation 12, 13. Indeed, ICV peaks early in life and once the skull sutures are completely fused, there is no further change in this measure, regardless of changes that may occur in brain tissue 14. In our study, we found significantly smaller ICV in FD patients, suggesting that in this condition a neurodevelopmental abnormality may be also present. It may be hypothesized that α-galactosidase A could play a role in the normal development of the CNS and its deficiency could lead to detectable neurodevelopmental abnormalities. These findings indicate that FD, once considered as an adult-onset condition, is a more complex phenomenon that encompass all ages, stressing the importance of a timely diagnosis and the importance of an early initiation of the enzyme-replacement therapy (ERT). Furthermore, we found no difference between FD and HC in terms of BPF and GMF, thus suggesting a harmonious volumetric reduction of all intracranial structures in FD patients, rather than a pure neurodegenerative phenomenon. Interestingly, in the post-hoc VBM analysis we found two clusters of reduced GM density involving both thalami with extension to the left hippocampus, in line with previous studies that demonstrated hippocampal atrophy in FD patients as a possible surrogate of primitive neuronal involvement, independent from brain vasculopathy 15, 16.Conclusion
In conclusion, we demonstrated that FD patients show an almost 10% reduction of the ICV compared to HC, with a preservation of BPF and GMF. Our results suggest that in FD patients an abnormality of brain development could be also present, thus emphasizing the importance of an early diagnosis of FD, and probably of an early ERT initiation.Acknowledgements
No acknowledgement found.References
1. Sweeley CC, Klionsky B. Fabry's disease: classification as a sphingolipidosis and partial characterization of a novel glycolipid. The Journal of biological chemistry 1963;238:3148-3150. 2. Germain DP. Fabry disease. Orphanet journal of rare diseases 2010;5:30. 3. Neufeld EF. Lysosomal storage diseases. Annual review of biochemistry 1991;60:257-280. 4. Staretz-Chacham O, Lang TC, LaMarca ME, Krasnewich D, Sidransky E. Lysosomal storage disorders in the newborn. Pediatrics 2009;123:1191-1207. 5. Fazekas F, Enzinger C, Schmidt R, et al. Brain magnetic resonance imaging findings fail to suspect Fabry disease in young patients with an acute cerebrovascular event. Stroke 2015;46:1548-1553. 6. Ashburner J, Friston KJ. Unified segmentation. NeuroImage 2005;26:839-851. 7. Malone IB, Leung KK, Clegg S, et al. Accurate automatic estimation of total intracranial volume: A nuisance variable with less nuisance. NeuroImage 2015;104:366-372. 8. Rudick RA, Fisher E, Lee JC, Simon J, Jacobs L. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. Multiple Sclerosis Collaborative Research Group. Neurology 1999;53:1698-1704. 9. Vågberg M, Granåsen G, Svenningsson A. Brain Parenchymal Fraction in Healthy Adults—A Systematic Review of the Literature. PLoS ONE 2017;12. 10. Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage 2007;38:95-113. 11. Ashburner J. Computational anatomy with the SPM software. Magnetic resonance imaging 2009;27:1163-1174. 12. Matsumae M, Kikinis R, Morocz IA, et al. Age-related changes in intracranial compartment volumes in normal adults assessed by magnetic resonance imaging. Journal of neurosurgery 1996;84:982-991. 13. Sgouros S, Goldin JH, Hockley AD, Wake MJ, Natarajan K. Intracranial volume change in childhood. Journal of neurosurgery 1999;91:610-616. 14. Morriss-Kay GM, Wilkie AO. Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. Journal of anatomy 2005;207:637-653. 15. Fellgiebel A, Wolf DO, Kolodny E, Müller MJ. Hippocampal atrophy as a surrogate of neuronal involvement in Fabry disease. Journal of Inherited Metabolic Disease 2012;35:363-367. 16. Lelieveld IM, Böttcher A, Hennermann JB, Beck M, Fellgiebel A. Eight-Year Follow-Up of Neuropsychiatric Symptoms and Brain Structural Changes in Fabry Disease. PLoS ONE 2015;10.Figures
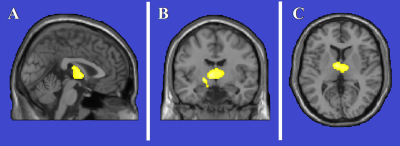
Figure
1.
Clusters of gray matter loss in Fabry Disease (FD) patients compared to healthy
controls (HC). Results are displayed for p < 0.05 FWE-corrected at cluster
level, and superimposed for anatomical reference to multiplanar reconstruction
of a single subject T1-weighted volume in the standard Montreal Neurological
Institute space (A: sagittal, B: coronal, C:axial). No region of increased gray
matter volume in FD patients compared to HC.