1797
ROTATING FRAME MRI CONTRASTS FOR ASSESSMENT OF WHITE MATTER ALTERATION IN MUCOPOLYSACCHARIDOSIS TYPE IAlena Svatkova1, Bryon A. Mueller2, Petr Bednařík3, Carol Nguyen1, Lubomír Vojtíšek4, Silvia Mangia3, Mikko Nissi5, Shalom Michaeli3, and Igor Nestrasil1
1Department of Pediatrics, University of Minnesota, Minneapolis, MN, United States, 2Department of Psychiatry, University of Minnesota, Minneapolis, MN, United States, 3Radiology, CMRR, University of Minnesota, Minneapolis, MN, United States, 4Central European Institute of Technology, Masaryk University, Brno, Czech Republic, 5Department of Applied Physics, University of Eastern Finland, Kuopio, Finland
Synopsis
Mucopolysaccharidosis type I (MPSI) is an inherited metabolic disease with severe and attenuated disease subtypes. While both MPSI subtypes manifest pronounced morphological brain changes, little has been discovered about alterations of white matter (WM) microstructure. Here, we utilized rotating frame MRI contrasts along with DTI to detect WM alterations between in 11 severe and 9 attenuated MPSI patients at 3T. T1ρ and RAFF4 detected WM differences between MPS subtypes that were not depicted by DTI. Outcomes demonstrate an exceptional sensitivity of rotating frame methods to probe WM microstructure in MPSI.
INTRODUCTION
Current development of novel brain-directed treatments that bypass the blood-brain barrier in Mucopolysaccharidosis type I (MPSI) has highlighted the need to establish sensitive MRI contrasts for comprehensive assessment of white matter (WM) deficits. Excessive intracellular accumulation of glycosaminoglycans due to inborn lysosomal enzymatic defect in MPSI affects the brain and leads to cognitive deficits.1,2 While histopathological studies and animal MPSI models detected WM abnormalities and congruently highlighted demyelination as a core of WM deficiency in MPS,1,3,4 little has been reported about WM damage in MPSI patients. Sparse DTI studies found alterations of diffusion parameters between MPSI patients and healthy individuals and ascribed those outcomes to neuro-inflammation and demyelination.5 However, only subtle DTI changes in WM of the corpus callosum (CC) were observed among MPSI subtypes, further underlying the unmet need to comprehensively characterize alterations of myelin content in MPSI patients of various severity.5,6 Innovative MRI method entitled, “Relaxation Along a Fictitious Field (RAFF) in the rotating frame of rank n (RAFFn)”, developed by our group,7,8 outperformed other MRI modalities including free precession T1 and T2, continuous wave T1ρ, and DTI methods in the sensitivity to myelin content in murine MPSI model.3 RAFF4 was therefore utilized here along with adiabatic T1ρ and DTI to probe WM alterations in MPSI patients.METHODS
Studies were conducted on a 3 T/ 90 cm bore Siemens Prisma scanner using a 32-channel RF head coil. Eleven severe MPSI-H (Hurler, 16.3±4.8 y/o) and 9 attenuated MPSI-A (Hurler-Scheie and Scheie, 18.9±7.5 y/o) patients participated in the study. For T1ρ and RAFF4 acquisitions, 30 slices were acquired with segmented GRE readout (4 segments), voxel size: 1.6x1.6x3.6 mm3, GRAPPA=3, TE=3.18 ms; TR=2 s. The parameters of the preparation pulses were used as described previously.7–9 Two diffusion MRI datasets were acquired with opposite phase encoding direction. Each diffusion dataset contained 17 diffusion-unweighted scans and 71 directions with b-values of 1000 and 2000mm/s2 with isotropic voxel size (1.5mm)3, TR=2720 s, TE=64ms; multiband (MB)=3, 90 slices. Anatomical T1- and T2-weighted scans were collected with isotropic voxel (0.8mm)3, TR/TE= 2400/2.62ms and TR/TE=3200/564ms, respectively. Anatomical and diffusion data were preprocessed using Human Connectome Project (HCP) pipeline and brain segmentation was carried out with FreeSurfer 5.3.0-HCP.10 Fractional anisotropy (FA) and mean diffusivity (MD) were calculated using DTIFIT tool in FSL.11 Rotating frame MRI and DTI maps were then aligned to the T1-weighted images using bb-register,12 and histograms were extracted from regions of interest (ROI) of CC and whole WM, which was further subdivided into cingulate, frontal, occipital, and parietal lobes. The whole WM ROI did not contain temporal WM because the T1ρ and RAFF4 acquisitions did not provide full coverage for all MPS subjects. Modes of the histograms for each ROI were estimated per subject, and compared among groups using two-tailed t-test. Bonferroni correction was used for multiple testing of the 4 lobar ROIs.RESULTS
Comparison between MPSI subtypes demonstrated statistically significant prolongation of T1ρ in all examined WM regions in severe MPSI-H relative to MPSI-A patients. RAFF4 was also prolonged in the occipital WM, whereas diffusion metrics failed to detect any significant changes. Representative parametric maps in MPSI subtypes are shown in Fig. 1, while the results of MRI measurements are summarized in Table 1 and Fig. 2.DISCUSSION
Our outcomes confirmed more profound WM deficits in MPSI-H compared to MPSI-A subtype, and corroborated initial DTI studies that demonstrated differences in WM composition between MPSI subtypes in CC.5,6 Alterations in rotating frame relaxation metrics between MPS subtypes may be ascribed to different disease phenotypes, as well as additional harmful effect of chemotherapy, applied prior hematopoietic stem cell transplantation that is utilized to treat MPSI-H but not MPSI-A.5 Results also align with murine MPS model that detected higher RAFFn values in affected animals and found close relationships between rotating frame relaxations and histological myelin density.3 Widespread T1ρ changes concomitant with RAFF4 abnormalities in the occipital lobe of MPSI–H suggest sensitivity of rotating frame contrasts to distinct pathophysiological features due to their inherent sensitivity to different motional regimes. The overall lengthening of T1ρ in WM of MPSI-H confirmed the pervasiveness of WM deficits in MPSI. Such findings are consistent with profound demyelination, axonal degeneration, gliosis, and neuro-inflammation in MPSI-H that were found in histopathological animal MPS studies.1CONCLUSION
Outcomes suggest that rotating frame MRI methods provide robust characterization of WM deficit in MPSI subtypes and are more sensitive to disease-related variations of WM microstructure than DTI metrics. Thus, RAFF and T1ρ methods show high promise to monitor disease- and treatment-related WM changes in clinical MPSI trials.Acknowledgements
MDBR-16-125-MPS, UL1TR000114, P41 EB015894, P30 NS076408, Marie Skłodowska-Curie grant, agreement #691110 (MICROBRADAM).References
[1] Wilkinson, F. L. et al. PLoS One 2012, 7 (4). [2] Shapiro, E. G. et al. Mol. Genet. Metab. 2015, 116 (1–2), 61–68. [3] Satzer, D. et al. PLoS One 2015, 10 (2), e0116788. [4] Tkac, I. et al. In ISMRM; Honolulu 2017, 2017. [5] Shapiro, E. et al. Mol. Genet. Metab. 2012, 107 (1–2), 116–121. [6] Svatkova, A. et al. In Organization for Human Brain Mapping; Geneva, 2016. [7] Liimatainen, T. et al. J. Magn. Reson. 2011, 209 (2), 269–276. [8] Liimatainen, T. et al. Magn. Reson. Med. 2015, 73 (1), 254–262. [9] Michaeli, S. et al. J. Magn. Reson. 2006, 181 (1), 135–147. [10] Glasser, M. F. et al. Neuroimage 2013, 80, 105–124. [11] Jenkinson, M. et al. Neuroimage 2012, 62 (2), 782–790. [12] Greve, D. N. et al. Neuroimage 2009, 48 (1), 63–72.Figures
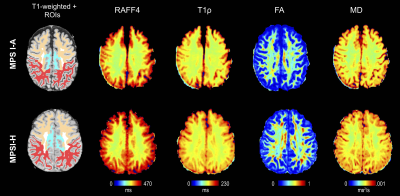
Figure 1 Representative maps
of relaxation time constants T1ρ, RAFF4, FA, and MD from one attenuated and
hurler MPSI subject. WM ROIs of interest visible on the selected slices are
also shown superimposed on the T1-weighted images, including frontal (orange), parietal (red) and cingulate WM (light-blue).
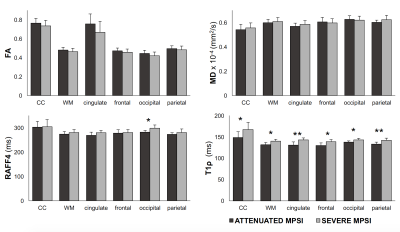
Figure 2 Group summaries for adiabatic T1ρ, RAFF4, FA, and MD for attenuated and severe MPSI subjects. Data shown as mean and SD. Asterisks (*) indicate p<0.05 and (**) indicate p<0.001, uncorrected. All results remain significant after multiple comparison correction.
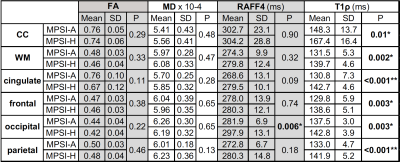
Table 1 Group means and standard deviations for adiabatic T1ρ, RAFF4, FA, and MD for attenuated (MPSI-A) and severe MPSI (MPSI-H) subjects. Asterisks (*) indicate p<0.05 and (**) indicate p<0.001, uncorrected. Results of lobar ROI analyses remain significant at multiple comparison corrected threshold of p<0.01.