1791
Regional CBF differences underlie neurocognitive outcomes in older children with congenital heart disease: a voxelwise mediation analysis1Radiology, Children's Hospital of Pittsburgh, Pittsburgh, PA, United States, 2Children's Hospital of Pittsburgh, Pittsburgh, PA, United States
Synopsis
We investigate in more detail the relationship between congenital heart disease (CHD), CBF, and neurocognitive outcome in older children by employing a novel voxelwise mediation analysis with CHD status the independent variable, NIH Toolbox scores the dependent variable, and voxelwise CBF the mediating variable. CHD patients display reduced CBF in the salience network (insula, medial prefrontal, caudate) which mediates lower performance on tests of memory and language function. However, the reduced CBF in the salience network mediates improved performance of executive function (flanker inhibitory control) likely due to less filtering out of presumed irrelevant but actually relevant information.
Introduction
Regionally-specific reductions in CBF have been previously seen in older children with congenital heart disease (CHD)1. CHD patients are at risk for neurocognitive deficits in various domains2, 3, and regional CBF differences have been shown to correlate with memory and language performance1. Here, we investigate in more detail the hypothesis that reduced regional CBF underlies poor neurocognitive outcome in CHD older children by employing a novel voxelwise mediation analysis.Methods
ASL: Pseudo-continuous ASL (pCASL) data was successfully acquired from 20 CHD patients (16 M, 4 F, age = 13.4 +/- 2.60 years) and 26 healthy controls (13 M, 13 F, age = 13.2 +/- 2.91 years). pCASL scan parameters were: labeling duration = 1500 ms, post-inversion delay = 1000 ms, pulse duration = 500 μs, 1 ms time between pulses; 2-D gradient-echo EPI acquisition, TR = 4000 ms, TE = 12 ms, matrix = 64 X 64, in-plane resolution = 4 mm2, slice thickness = 4 mm, SENSE factor = 2. 45 tagged and 45 control acquisitions were acquired for total scan time = 6 min.
Neurocognitive: Participants were administered the cognitive portion of the NIH Toolbox4. The battery incorporates tests of executive function, memory (episodic and working), language, and processing speed.
ASL Pre-processing: All analysis was performed using SPM8, FSL, and in-house routines in IDL. The following procedures were used to minimize the risk of spurious results due to participant motion and spatial misregistration. For each participant, the pCASL datasets were coregistered to correct for motion using an affine transform. Control-tag and average control images were computed using a voxelwise GLM, with motion and drift parameters included as nuisance covariates. Absolute CBF was estimated from the fractional difference using the two-compartment model5 with literature values6. The reference image was skull-stripped, and segmented into GM, WM, and CSF in native space. The GM image was spatially normalized into MNI space using the gray matter template in SPM8; a study-specific GM template was obtained by averaging across participants and the spatial normalization repeated, with the CBF images transformed into MNI space using the same transformation. CBF and GM maps were spatially filtered using a Gaussian filter with σ = 3 mm.
Voxelwise analysis: Analyses (GLM or mediation) were performed only on participants with rms translational motion < 1 voxel and only on voxels with GM probability > 78%. Covariates of no interest are age, sex, rms motion, and GM probability (different for each voxel). A voxelwise GLM was performed comparing CHD to controls. Additionally, for each of the NIH Toolbox subtests, a voxelwise mediation analysis was performed with CHD status the independent variable, voxelwise CBF the mediator, and NIH Toolbox score the dependent variable. Statistical significance was obtained via bootstrapping (5,000 samples) with bias-corrected and accelerated confidence intervals7, 8. Results were deemed significant at FWE-corrected p < 0.05, as determined via Monte Carlo analysis9.
Results
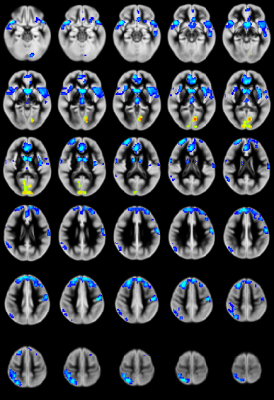
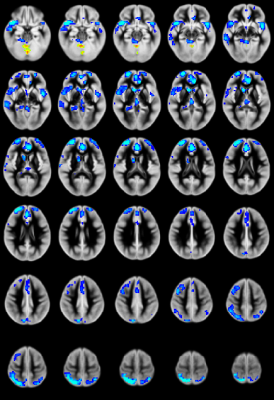
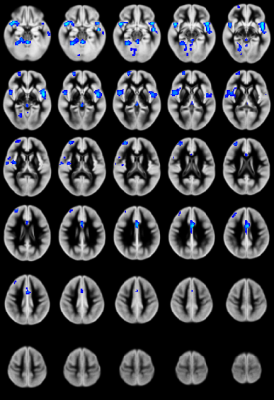
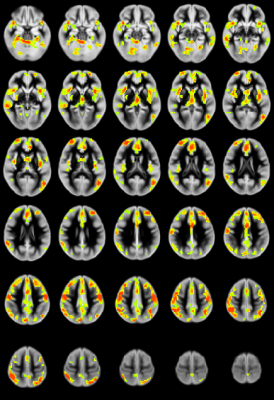
CHD patients display reduced CBF primarily in the salience network (insula, caudate, putamen, medial prefrontal inferior parietal lobule) and increased CBF in medial occipital areas (Figure 1). CBF differences mostly in the salience network mediate reduced neurocognitive outcome for memory (as shown via the picture sequence memory (PSM) subtest; Figure 2) and language (as shown via the picture vocabulary (PV) subtest; Figure 3). However, CBF differences in the salience network and default mode network mediate improved outcome for executive function (as shown via the flanker inhibitory control (FIC) subtest; Figure 4).Discussion
Our results show that CBF reductions in CHD patients occur on a regional and not a global basis (mainly in the salience network). Additionally, the increased CBF in occipital regions contradicts the widely-held view that the effect of CHD is a global reduction of CBF. Mediation analyses are a stronger (though not absolutely determinative) indicator of causality as compared to simple correlations when direct manipulation of the independent variable or the mediator is not possible7. The regional CBF reduction appears to impact neurocognitive performance in domains which require the salience network, which is necessary for concentration on relevant stimuli and filtering out of irrelevant ones. Consistent with this, CBF reductions in the salience network mediate poorer memory and language performance. However, these same reductions mediate improved executive function performance in the FIC test, in which seemingly “irrelevant” stimuli are in fact relevant.Conclusion
A voxelwise mediation analysis was performed using pCASL data obtained from CHD older children and normal controls. It was found that CBF reductions in the salience network mediate poorer neurocognitive outcomes in memory and language function.Acknowledgements
No acknowledgement found.References
1. Schmithorst VJ, Panigrahy A, ISMRM Annual Meeting, Honolulu, HI, 901, 2017.
2. Hoffman GM et al., J Thorac Cardiovasc Surg, 146, 1153-64, 2013.
3. Mussatto KA et al., Pediatrics, 133, e570-7, 2014.
4. Gershon RC et al., Neurology, 80, S2-6, 2013.
5. Alsop DC, Detre JA, J Cereb Blood Flow Metab, 16, 1236-49, 1996.
6. Schmithorst VJ et al., J Magn Reson Imaging, 39, 1104-17, 2014.
7. Hayes AF, Scharkow M, Psychol Sci, 24, 1918-27, 2013.
8. DiCiccio T, Efron B, Statistical Science, 13, 189-228, 1996.
9. Ledberg A et al., Neuroimage, 8, 113-28, 1998.
Figures