1790
Regional Brain Myelin Changes in Patients with Single Ventricle Heart Disease1Department of Anesthesiology, University of California Los Angeles, Los Angeles, CA, United States, 2UCLA School of Nursing, University of California Los Angeles, Los Angeles, CA, United States, 3Division of Pediatric Cardiology, University of California Los Angeles, Los Angeles, CA, United States, 4Division of Pediatric Cardiology, Children’s Hospital Los Angeles, Los Angeles, CA, United States, 5Department of Radiological Sciences, University of California Los Angeles, Los Angeles, CA, United States, 6Department of Bioengineering, University of California Los Angeles, Los Angeles, CA, United States, 7Brain Research Institute, University of California Los Angeles, Los Angeles, CA, United States
Synopsis
Single ventricle heart disease (SVHD) subjects show brain injury in multiple gray and white matter based on MRI procedures. However, the extent of regional myelin integrity in SVHD is unclear. We examined the regional brain myelin integrity in SVHD adolescents using the ratio of T1-weighted and T2-weighted MRI signal intensity, and found decreased values in critical autonomic, mood, and cognitive control sites, functions that are deficient in the condition, likely resulting from hypoxic/ischemic processes.
Introduction
Single ventricle heart disease (SVHD) subjects show brain injury as evaluated by various MRI procedures, which includes gray and white matter volume loss, ischemic infarcts, and focal or multifocal abnormalities, preoperatively and postoperatively.1-3 However, the extent of myelin changes in subcortical and white matter brain areas in SVHD remains unclear. Although various MRI methods, including T2-relaxometry, magnetization transfer imaging, and diffusion tensor imaging-based fractional anisotropy and mean diffusivity procedures can demonstrate the extent of myelin changes, these methods have either poor spatial resolution or require long data acquisition times and specialized image processing skills. However, it has been proposed that the ratio of T1-weighted and T2-weighted MRI signal intensity can increase the sensitivity in detecting myelin‐related changes in subcortical and white matter brain areas and requires less data acquisition time, and also enhances the contrast-to-noise ratio.4 We aimed here to examine the regional brain myelin integrity in SVHD over control subjects using T1- and T2-weighted images. We hypothesized that regional myelin integrity will be reduced in autonomic, mood, and cognitive controls sites in SVHD, functions that are deficient in the condition, compared to control subjects.Materials and Methods
Twenty SVHD (age, 15.8±1.3 years; body-mass-index (BMI), 21.9±4.9 kg/m2; 11 male) and 30 control adolescents (age, 15.9±1.2 years; BMI, 21.8±4.0 kg/m2; 15 male) participated in this study. Control subjects were healthy, without any history of chronic medical or psychiatric conditions or head injury, and were recruited from the local area. SVHD subjects, who have undergone surgical palliation with Fontan completion, were recruited via flyers or referrals from pediatric cardiology clinics, and private practice cardiology groups. All procedures were approved by the Institutional Review Boards and each subject provided written informed consent prior to the study. Brain MRI scans were acquired using a 3.0-Tesla MRI scanner (Magnetom Prisma, Siemens, Erlangen, Germany). High-resolution T1-weighted images were acquired using a magnetization-prepared rapid gradient echo sequence (MPRAGE) sequence (TR = 2200 ms; TE = 2.4 ms; inversion time = 900 ms; flip angle (FA) = 9°; matrix size = 320×320; field of view (FOV) = 230×230 mm2; slice thickness = 0.9 mm; number of slices = 192). T2-weighted images were collected using a spin-echo pulse sequence in the axial plane (TR = 10,000 ms; TE = 124 ms; FA = 130⁰; matrix size = 256×256; FOV = 230×230 mm2; slice-thickness = 3.5 mm). We realigned and averaged both T1-weighted images to remove any potential variation between scans. T2-weighted and mean T1-weighted images were bias-corrected, mean T1-weighted images were co-registered and resliced to their corresponding T2-weighted images, and then myelin maps were generated by dividing the T1-weighted images with T2-weighted images. We normalized the myelin maps to Montreal Neurological Institute space and smoothed (Gaussian kernel, 8 mm). The smoothed myelin maps were compared voxel-by-voxel between groups using ANCOVA (SPM12; covariates: age, sex; uncorrected threshold p < 0.001). Brain clusters with significant differences between groups were overlaid onto background images for structural identification.Results
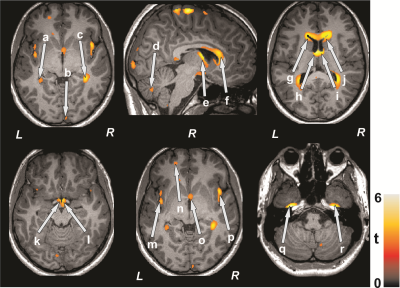
No significant differences in age (p = 0.78), gender (p = 0.74), or BMI (p = 0.97) appeared between groups. Multiple brain areas in SVHD adolescents showed decreased myelin integrity compared to control subjects (Figure 1). Brain sites that showed decreased myelin integrity in SVHD adolescents included the para-hippocampal gyrus (a, c), occipital cortices (b), cerebellar cortices (d), fornix (e, o), anterior, mid, and posterior corpus callosum (f), caudate nuclei (g, j), thalamus (h, i), hypothalamus, mammillary bodies (k, l), insular cortices (m, p), prefrontal cortices (n), and temporal cortices (q, r).Discussion
Regional brain myelin integrity is significantly decreased in multiple brain sites in SVHD over control adolescents. These brain regions with myelin changes are associated with autonomic, mood, and cognition regulation, functions that are compromised in SVHD subjects. These findings may result from delayed brain development and/or hypoxic/ischemic induced processes in the condition.Conclusions
The findings indicate that myelin changes occur in SVHD adolescents in various sites, which can be assessed with the ratio of T1- and T2-weighted images.Acknowledgements
This work was supported by National Institutes of Health R01-NR013930 and R01-NR016463.References
1. Von Rhein M, Scheer T, Loenneker T, et al. J Pediatr. 2011; 158: 984-9.
2. Von Rhein M, Buchmann A, Hagmann C, et al. Brain. 2014; 137: 268-76.
3. Rollins CK, Watson CG, Asaro LA, et al. J Pediatr. 2014; 165:936-944.
4. Ganzetti M, Wenderoth N, Mantini D, et al. Front Hum Neurosci. 2014; 8:671.