1788
Assessing white matter development in peri-pubertal children using longitudinal fixel-based analysis1Department of Paediatrics, The University of Melbourne, Parkville, Australia, 2Developmental Imaging, Murdoch Childrens Research Institute, Parkville, Australia, 3The Florey Institute of Neuroscience and Mental Health, Heidelberg, Australia, 4Clinical Sciences, Murdoch Childrens Research Institute, Parkville, Australia, 5The Royal Children's Hospital, Parkville, Australia, 6Judith Lumley Centre, La Trobe University, Melbourne, Australia
Synopsis
Recent evidence suggests that the pubertal period corresponds with changes to white matter microstructure above and beyond age-related development. This study uses a longitudinal fixel-based analysis to investigate which regions of the brain correspond to changes in white matter fibre density and cross-section during pubertal development. We show that, over a 16-month follow-up period, increases in fibre density and cross-section are predominantly in the posterior white matter. These results add to evidence that white matter develops in a posterior-anterior fashion, and signifies the dynamic nature of brain development during puberty.
Introduction
White matter development is thought to follow a posterior to anterior maturation trend over childhood and adolescence, evidenced by histological1 and diffusion tensor imaging2,3 studies. Recent work suggests the pubertal period is accompanied by remodelling of white matter4,5, likely due to the surge in circulating adrenal hormones that can cross the blood brain barrier. This study investigated longitudinal white matter fibre development in children on the cusp of pubertal onset. We applied fixel-based analysis (FBA)6, a recently developed white matter imaging framework, in order to estimate fibre density (FD), which is proportional to intra-axonal volume, fibre cross-section (FC) and fibre density and cross-section (FDC).Methods
Diffusion-weighted imaging (DWI) data were acquired for 60 typically developing children (27 female) on a 3.0 T Siemens Tim Trio (b=2800s/mm2, 60 directions, 2.4x2.4x2.4mm voxel size, TE/TR=110/3200ms, multi-band factor=3). Data were acquired at two time-points approximately 16 months apart: time1 (M = 10.4, SD = .41 years old), time2 (M = 11.7, SD = .47 years old). Children were classified peri-pubertal as 43% had physical signs of pubertal onset at time1, and 70% at time2.
DWI data were processed using MRtrix3 (v3.0rc1) using a recommended pipeline6. An unbiased longitudinal fibre orientation distribution (FOD) template was generated across the two time-points. Longitudinal permutation-based testing of FBA metrics was performed using connectivity-based fixel enhancement7 with a paired t-test design. Significant regions were identified at pFWE < .05.
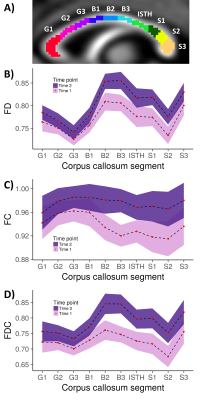
Further post-hoc statistical testing of the corpus callosum was performed via segmentation into 10 subregions: the genu (G1, G2, G3); body (B1, B2, B3); isthmus (ISTH); and splenium (S1, S2, S3) as previously described8 (Fig 3a). The mean FD, FC and FDC value was computed for each callosal subregion.
Results
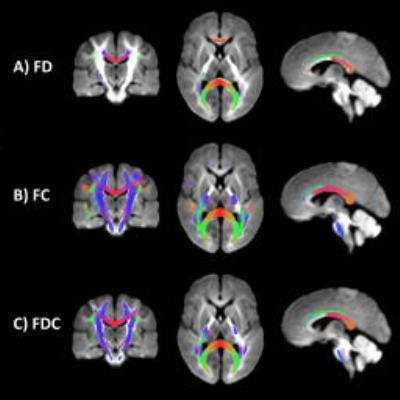
We observed a statistically significant increase in FD over time (pFWE < .05; Fig 1a) localised to the mid and posterior corpus callosum, namely G1, B2, B3, ISTH, splenium and its bilateral projections (forceps major), as well as left anterior cingulum and left superior longitudinal fasciculus (SLF). The strongest evidence for change was in forceps major and left SLF. Increases in FC (pFWE < .05; Fig 1b) were more widespread compared with that observed in FD (Fig 1b). Increases were localised to the full extent of the corpus callosum apart from G1 and G2, as well as bilateral projections of the callosal subregions, left cingulum, bilateral SLF and bilateral cortico-spinal tract (CST). Increases in FDC (pFWE < .05; Fig 1c-2) were localised to the full extent of the body, isthmus and splenium, left cingulum, bilateral SLF and CST.
Post-hoc analysis of the corpus callosum subregions revealed an upward translation of the FD, FC and FDC profiles with time (Fig 3b-d). Non-overlapping confidence intervals (indicating statistical significance at p < .01) were observed in the posterior body and anterior splenium, consistent with the whole-brain findings.
Discussion
The current study is the first known application of FBA in a longitudinal experiment. The observed increases in fibre density and cross-section longitudinally might be reflected by increasing axonal diameter, or axon count, during the 16-month follow-up period. We have previously shown that pubertal children have greater FD in the posterior splenium compared with age-matched pre-pubertal children4 using the same group of children. Subsequently we hypothesised that longitudinal maturation of the white matter would persist anteriorly towards the midbody.
In line with our predictions, predominately the posterior white matter exhibited increased FD, FC and FDC longitudinally. Post-hoc analysis of the corpus callosum revealed that the most evidence for a difference over time was observed in the anterior splenium and body of the corpus callosum. Taken with our previous findings, this study adds to a growing body of evidence that white matter, at least in the corpus callosum, develops in a posterior-anterior fashion, and that pubertal onset and progression may contribute to this gradient.
We also observed increases in bilateral CST and SLF, consistent with previous studies that link pubertal progression with the development of these white matter pathways2,9. We were unable to fully model other factors such as sex and pubertal stage using the current statistical framework. Future work should explicitly investigate whether sex, and/or the rate of pubertal progression, affects fibre development above and beyond age-related development.
Conclusion
We present evidence that white matter maturation around the cusp of pubertal onset primarily occurs in the central and posterior white matter. Using a novel longitudinal fixel-based analysis framework, we demonstrated that apparent fibre density and fibre cross-section increased within a 16-month scan rescan period. These results highlight the dynamic nature of brain development during the time of pubertal onset.Acknowledgements
This research was conducted within the Developmental Imaging research group, Murdoch Children's Research Institute and the Children’s MRI Centre, The Royal Children's Hospital, Melbourne, Victoria. It was supported by the Murdoch Children's Research Institute, The Royal Children’s Hospital, Department of Paediatrics at The University of Melbourne, and the Victorian Government's Operational Infrastructure Support Program.References
1. Yakovlev, P.I., Lecours, A.R., 1967. The myelogenetic cycles of regional maturation of the brain. In: Minkowski, A (Ed.), Regional Development of the Brain Early in Life. Blackwell Scientific Publications Inc., Boston, pp. 3–70. Massachusetts.
2. Asato, M.R., et al., White Matter Development in Adolescence: A DTI Study. Cerebral Cortex, 2010. 20(9): p. 2122-2131.
3. Lebel, C., et al., Microstructural maturation of the human brain from childhood to adulthood. NeuroImage, 2008. 40(3): p. 1044-1055.
4. Genc, S., et al., White matter alterations at pubertal onset. Neuroimage, 2017. 156: p. 286-292.
5. Herting, M.M., et al., Longitudinal changes in pubertal maturation and white matter microstructure. Psychoneuroendocrinology, 2017. 81: p. 70-79.
6. Raffelt, D.A., et al., Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage, 2017. 144(Pt A): p. 58-73.
7. Raffelt, D.A., et al., Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. Neuroimage, 2015. 117: p. 40-55.
8. Genc, S., et al., Age, sex, and puberty related development of the corpus callosum: a multi-technique diffusion MRI study. bioRxiv 187724, 2017.
9. Pangelinan, M.M., et al., Puberty and testosterone shape the corticospinal tract during male adolescence. Brain Structure & Function, 2016. 221(2): p. 1083-1094.
10. Aboitiz, F., et al., Fiber composition of the human corpus callosum. Brain Res, 1992. 598(1-2): p. 143-53.
Figures