1787
Motor connectivity of the midbrain in healthy children defined using connectivity based parcellation1Developmental Imaging and Biophysics Section, UCL GOS Institute of Child Health, University College London, London, United Kingdom, 2UCL Ear Institute, University College London, London, United Kingdom, 3Cognitive Neuroscience and Neuropsychiatry Section, UCL GOS Institute of Child Health, University College London, London, United Kingdom
Synopsis
Delineation of midbrain regions connected with the motor cortex may be useful in evaluating disruptions of motor pathways in paediatric patients. We used the established winner-takes-it-all method to parcellate the midbrain according to cortical connectivity in healthy children aged 6-12 years. The percentage of ipsilateral midbrain occupied by motor parcels was negatively associated with age on the right side only, producing an association between age and interhemispheric asymmetry. Our findings indicate that age and interhemispheric differences need to be taken into account if this method is to be utilised for quantitative comparisons of midbrain-motor connectivity in children.
Introduction
The midbrain is a site of confluence of several pathways crucial for motor function that are not easily discernable on conventional MRI in children. Asymmetry measures of the anterior midbrain and brainstem are related to severity of motor impairment in children with congenital hemiplegia1, 2. In the more acute setting, diffusion weighted MRI signal changes in the cerebral peduncles are predictive of motor outcome in neonatal and paediatric stroke3-6. Defining midbrain regions that exhibit high connectivity with motor cortex could potentially enable more specific assessment of motor pathway disruption in relation to injury.
We aimed to investigate the feasibility of segmenting the whole midbrain in healthy children aged 6-12 years using the well-established winner-takes-it-all connectivity based parcellation7. Our second aim was to explore whether the size of resulting midbrain motor parcels was associated with age.
Methods
T1 MPRAGE (voxel size 1 × 1 × 1 mm3) and multi-shell diffusion (60 directions at b=1000 s/mm2, 60 directions at b=2200 s/mm2; voxel size 2 × 2 × 2 mm3, 0.2 mm slice gap) imaging data of 33 healthy children aged 6-12 years (17 male; 5 left handed; mean age = 9.0 years, SD = 1.7) acquired on the same 3T MRI Siemens Prisma system were analysed.
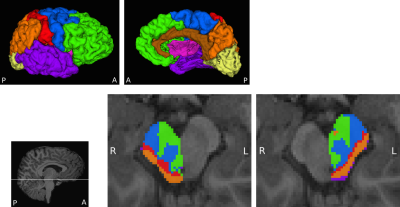
The midbrain and eight cortical masks per hemisphere were defined in T1-weighted images of each participant using Freesurfer8,9. Midbrain masks were manually edited and split into right and left halves. Cortical masks included prefrontal, motor, somatosensory, posterior parietal, temporal, occipital, cingulate and insula regions (Figure 1). The large motor area consisted of the precentral gyrus, paracentral lobule, Brodmann Area 6 (BA6) and caudal middle frontal cortex to encompass primary, pre- and supplementary motor cortices.
Diffusion datasets were distortion and motion corrected with FSL TOPUP and eddy10. Up to 3 fibres were fitted in each voxel with FSL BedpostX using the multi-shell model11. Probabilistic tractography was carried out in FSL ProbtrackX. 5000 samples were seeded from right and left midbrain, with eight ipsilateral cortical areas as targets and bilateral cortical ribbon and the corpus callosum as termination masks. All masks were supplied in native T1 space, together with a transform to diffusion space obtained using FSL FLIRT, resulting in ProbtrackX outputs being in native T1 space. Every midbrain voxel was subsequently labelled according to the ipsilateral cortical area receiving the highest proportion of samples from that voxel, thus parcellating the midbrain7.
Volumes of midbrain-motor area connectivity
defined parcels (MA CDPs) were extracted, and the percentage of ipsilateral
midbrain they occupy calculated (MA CDP%). An asymmetry index (AI) was
calculated for each participant ((left MA CDP% / right MA CDP%) ×
100). All MA CDPs were registered to a study
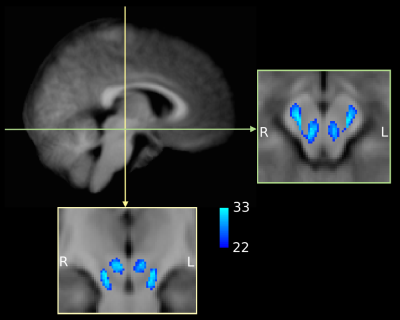
specific T1 template to visually inspect inter-subject spatial consistency (Figure 2).
Results
The midbrain was successfully parcellated in all participants. MA CDPs appear to consist of a lateral portion located in the cerebral peduncles, and a medial portion located in the tegmentum (Figure 1). The topography of CDPs in cerebral peduncles is consistent with similar parcellations in adults12,13. The tegmental portion of MA CDPs was present in all participants, although variable in size and shape. Group overlap maps show considerable inter-subject consistency (Figure 2).
Overall, MA CDP% values did not differ between the right (mean = 40.1%, SD = 7.72) and left (mean = 38.3%, SD = 7.93) hemispheres (t(32) = 1.48, p = 0.15). A significant negative correlation was found between age and right-sided MA CDP% (Pearson’s r = −.425, p = .014), while there was no linear association on the left side (Pearson’s r = −.066, p = .716). Consequently, age also correlated with the AI of MA CDP% (Pearson’s r = .381, p= .029).
Discussion
Decreasing MA CDP% with age on the right side only may reflect interplay between different maturational rates of various white matter pathways, and their interhemispheric differences, that are detectable on diffusion imaging during childhood14. However, our study is limited by the relatively small sample size and cross sectional design. Validation is needed in a larger study population with longitudinal measures, which would allow assessment of more complex relationships with age and potential interactions with other factors.Conclusion
Connectivity based winner-takes-it-all parcellation can be used to define midbrain segments with high connectivity to motor cortical areas in children aged 6-12 years. Our study indicates that any comparisons of resulting midbrain motor parcels should take into account age and interhemispheric differences, but a replication of our findings is required.Acknowledgements
NIHR Great Ormond Street Hospital Biomedical Research Centre
Action Medical Research
Child Health Research Appeal Trust
The Waterloo Foundation
References
1. Duque J, Thonnard JL, Vandermeeren Y, et al. Correlation between impaired dexterity and corticospinal tract dysgenesis in congenital hemiplegia. Brain. 2003;126(3):732–47.
2 Bouza H, Dubowitz LM, Rutherford M, Pennock JM. Prediction of outcome in children with congenital hemiplegia: a magnetic resonance imaging study. Neuropediatrics. 1994;25(2):60–6.
3. De Vries LS, Van der Grond J, Van Haastert IC, Groenendaal F. Prediction of outcome in new-born infants with arterial ischaemic stroke using diffusion-weighted magnetic resonance imaging. Neuropediatrics. 2005;36(1):12–20.
4. Groenendaal F, Benders MJ, De Vries LS. Pre-Wallerian degeneration in the neonatal brain following perinatal cerebral hypoxia-ischemia demonstrated with MRI. Semin Perinatol. 2006;30(3):146–50.
5. Kirton A, Shroff M, Visvanathan T, DeVeber G. Quantified corticospinal tract diffusion restriction predicts neonatal stroke outcome. Stroke. 2007;38(3):974–80.
6. Domi T, Deveber G, Shroff M, et al. Corticospinal tract pre-wallerian degeneration: A novel outcome predictor for pediatric stroke on acute MRI. Stroke. 2009;40(3):780–7.
7. Behrens TEJ, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6(7):750–7.
8. Klein A, Tourville J. 101 labeled brain images and a consistent human cortical labeling protocol. Front Neurosci. 2012;6:171.
9. Iglesias JE, Van Leemput K, Bhatt P, et al. Bayesian segmentation of brainstem structures in MRI. Neuroimage. 2015;113:184–95.
10. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–78.
11. Jbabdi S, Sotiropoulos SN, Savio AM, et al. Model-based analysis of multishell diffusion MR data for tractography: How to get over fitting problems. Magn Reson Med. 2012;68(6):1846–55.
12. Ramnani N, Behrens TEJ, Johansen-Berg H, et al. The evolution of prefrontal inputs to the cortico-pontine system: Diffusion imaging evidence from macaque monkeys and humans. Cereb Cortex. 2006;16(6):811–8.
13. Lazar M, Alexander AL. Bootstrap white matter tractography (BOOT-TRAC). Neuroimage. 2005;24(2):524–32.
14. Krogsrud SK, Fjell AM, Tamnes CK, et al. Changes in white matter microstructure in the developing brain - A longitudinal diffusion tensor imaging study of children from 4 to 11 years of age. Neuroimage. 2016;124:473–86.
Figures