1785
Paediatric brain tissue properties measured with magnetic resonance elastography1Neuroscience Research Australia, Randwick, NSW, Australia, 2University of New South Wales, School of Medical Sciences, Kensington, NSW, Australia, 3University of New South Wales, Prince of Wales Clinical School, Kensington, NSW, Australia
Synopsis
Magnetic resonance (MR) elastography is a technique to noninvasively measure the mechanical properties of soft tissues. While adult brain data obtained with MR elastography is readily available, there is little data for healthy paediatric brains throughout development. MR elastography was performed on 25 healthy paediatric subjects aged between 7-18 years at three frequencies, and the shear moduli of white and grey matter were calculated and compared to data obtained from 10 healthy adults. The shear modulus of paediatric brains was not found to be age dependent, with no significant differences between adult and paediatric brains.
Introduction
Magnetic resonance (MR) elastography is a technique to noninvasively measure the mechanical properties of soft tissues. Accurate mechanical properties of healthy tissues are needed for a variety of applications, including models and simulations for prediction of injuries, and as a baseline for comparison to pathological states.
While properties of the adult brain have been previously investigated 1,2, there is little data available on the properties of human paediatric brains throughout development. Previous studies on animal models using mechanical testing 3 and MR elastography 4 suggest significant differences between immature and mature brain tissue, and MR elastography of human paediatric liver has shown lower stiffness values compared to adults.5
Brain remodelling of white and grey matter from childhood to adulthood has been extensively documented in scientific literature 6,7 and in this study we aimed to determine whether their mechanical properties also change throughout development.
Methods
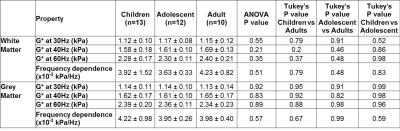
13 healthy children aged between 7-12 years (7 boys, 6 girls), 12 adolescents aged 13-18 years (5 boys, 7 girls), and 10 adults aged between 23-44 years (5 men, 5 women) were scanned in a clinical 3T MRI scanner (Philips AchievaTX).
The MR elastography setup consisted of a set of actuator coils connected to a bite bar and mouthguard, which transferred vibration to the participant’s brain.1 Anatomical and elastography data were acquired transversely at the level of the corpus callosum, and elastography data was collected at three frequencies- 30Hz, 40Hz, and 60Hz to investigate the frequency dependence.
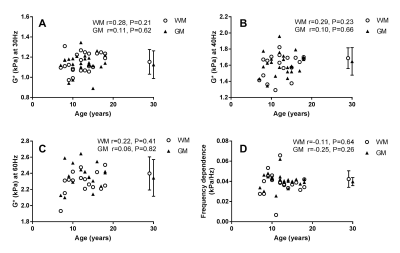
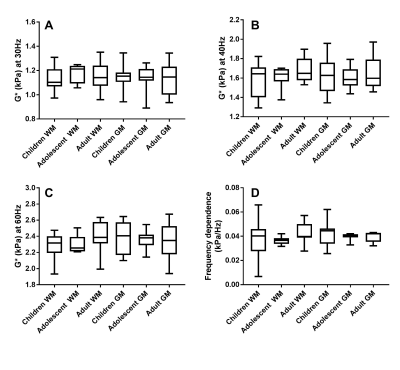
Regions of interest for white matter (WM) and grey matter (GM) were manually drawn from anatomical images, and the complex shear modulus G* was calculated with previously validated custom software.1,8 Age dependence of G* during development was assessed using Spearman correlations on the data from children and adolescents for each region of interest. One way analysis of variance (ANOVA) and Tukey’s multiple comparisons tests were also performed to compare these to the adult data.
Results
Brain stiffness values were found not to be significantly correlated with age for white matter at 30Hz, 40Hz and 60Hz (Spearman correlation r=0.28 [P=0.21], r=0.29 [P=0.23], r=0.22 [P=0.41] respectively), and likewise for grey matter (Spearman correlation, r=0.11 [P=0.62], r=0.10 [P=0.66], r=0.06 [P=0.82] respectively). Similarly, the frequency dependence was not significant. (Spearman correlation WM r=-0.11 [P=0.64], GM r= -0.25 [P=0.26]). Comparisons of adult to paediatric data also showed no significant differences of G* between groups (Table 1).Conclusion
The shear modulus measured in healthy paediatric subjects was not found to be significantly correlated with age for both white and grey matter, and comparisons to adult data showed no significant differences.Acknowledgements
This research was funded by an Australian Research Council (ARC) Discovery grant. L.E.B. is supported by an Australian National Health and Medical Research Council (NHMRC) senior research fellowship.References
1. Green, M.A., Bilston, L.E., and Sinkus, R., In vivo brain viscoelastic properties measured by magnetic resonance elastography. NMR Biomed, 2008. 21(7): p. 755-64.
2. Sack, I., Beierbach, B., Hamhaber, U., Klatt, D., and Braun, J., Non-invasive measurement of brain viscoelasticity using magnetic resonance elastography. NMR Biomed, 2008. 21(3): p. 265-271.
3. Gefen, A., Gefen, N., Zhu, Q., Raghupathi, R., and Margulies, S.S., Age-dependent changes in material properties of the brain and braincase of the rat. J Neurotraum, 2003. 20(11): p. 1163-77.
4. Pong, A.C., Jugé, L., Cheng, S., and Bilston, L.E., Longitudinal measurements of postnatal rat brain mechanical properties in-vivo. J Biomech, 2016. 49(9): p. 1751-1756.
5. Etchell, E., Jugé, L., Hatt, A., Sinkus, R., and Bilston, L.E., Liver Stiffness Values Are Lower in Pediatric Subjects than in Adults and Increase with Age: A Multifrequency MR Elastography Study. Radiology, 2016. 283(1): p. 222-230.
6. Lebel, C., Walker, L., Leemans, A., Phillips, L., and Beaulieu, C., Microstructural maturation of the human brain from childhood to adulthood. Neuroimage, 2008. 40(3): p. 1044-55.
7. Giedd, J.N., Blumenthal, J., Jeffries, N.O., Castellanos, F.X., Liu, H., Zijdenbos, A., Paus, T., Evans, A.C., and Rapoport, J.L., Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci, 1999. 2(10): p. 861-863.
8. Sinkus, R., Siegmann, K., Xydeas, T., Tanter, M., Claussen, C., and Fink, M., MR elastography of breast lesions: understanding the solid/liquid duality can improve the specificity of contrast-enhanced MR mammography. Magn Reson Med, 2007. 58(6): p. 1135-44.
Figures