1779
Intraoperative Volatile Anesthetic Exposure Predicts Reduced Frontal Lobe Connectivity Compared to Dexmedetomidine in Infants with Congenital Heart Disease1Radiology, University of Pittsburgh, Pittsburgh, PA, United States, 2Anesthesiology, Children's Hospital of Pittsburgh UPMC, Pittsburgh, PA, United States, 3Science Technology and Mathematics, Regent University, Pittsburgh, PA, United States, 4Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA, United States, 5Critical Care Medicine, Children's Hospital of Pittsburgh of UPMC, Pittsburgh, PA, United States, 6Radiology, Children's Hospital of Pittsburgh of UPMC, Pittsburgh, PA, United States
Synopsis
Anesthetic neurotoxicity in infants with repetitive exposure is a risk factors for adverse neurodevelopmental outcomes. Dexmedetomidine exposure is thought to have neuroprotective effects. We tested the hypothesis that intraoperative volatile anesthetic exposure is predictive of aberrant brain connectivity in the post-operative period in CHD infants, relative to dexmedetomidine exposure using DTI and BOLD imaging. Using both hypothesis driven and data driven approaches, as well as graph analysis we showed that Increased volatile anesthetic exposure in the intraoperative period is associated with reduced post-operative frontal brain connectivity in CHD infants, while DEX exposure was associated with metrics of improved brain connectivity.
INTRODUCTION:
The mechanism underlying anesthetic neurotoxicity in infants with repetitive exposure is one of the most significant unanswered questions in pediatric anesthesia despite recent FDA warning. Extensive animal data, including primates suggest that apoptosis and aberrant brain connectivity likely underlies poor neurocognitive outcomes. Significant epidemiologic human data especially prolonged and/or repeated exposures early in life. Anesthetic/sedative techniques have recently been described as risk factors for adverse neurodevelopmental outcomes for infants undergoing cardiac surgery.1 Dexmedetomidine exposure is thought to have neuroprotective effects. We tested the hypothesis that intraoperative volatile anesthetic exposure is predictive of aberrant brain connectivity in the post-operative period in CHD infants, relative to dexmedetomidine (DEX) exposure.METHODS:
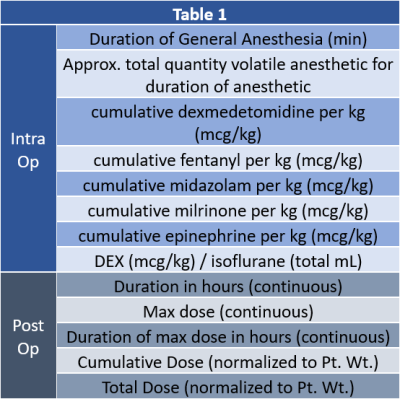
A total of 85 term neonates with CHD were recruited prospectively from the fetal period for pre-operative and post-operative imaging, with 44 of the patients (PCA of 46 ± 5.6 weeks at scan) completing post-op MRI scans. Participants were scanned on a Siemens 3T Skyra system using a 32-channel head coil – each receiving a DTI with the following parameters: FOV = 256 mm, voxel size = 2.0mm (isotropic), TE/TR=92ms/12600 ms, and 42-directions at B=1000s/ mm2. DEX and anesthetic clinical factors, during the intra-op and post-op periods, examined in this study are presented in Table 1 (Figure 1). Both hypothesis and data-driven approaches were used to analyze the relationship between intraoperative volatile anesthetic and intraoperative/postoperative DEX exposure and post-operative brain structural (DTI) and functional (Resting BOLD) connectivity. As part of the hypothesis driven analysis, manual tractography was performed using DSI studio2 for the following tracts: genu, body, and splenium of the corpus callosum (CC); left and right of cortico-spinal tract (CST), fronto-occipital fasciculus (IFOF), inferior (ILF) and superior (SLF) longitudinal fasciculi. For data driven voxel-based analysis the DTI were preprocessed for eddy current correction3 and computation of DTI metrics were performed using routines in FSL.4 Spatial normalization was performed using routines in SPM8.5 The L1 images were normalized to a neonatal template and the FA images were normalized to the standard space using that transformation. A study-specific template was constructed via averaging the FA images and then co-registering to the neonatal white matter template. For graph analysis, the study-specific template was back normalized to the individual subject's native space and the neonatal parcellation atlas was transformed into native space using that transformation. Graph metrics were computed using routines in Brain Connectivity Toolbox (BCT)6 and IDL (Exelis Visual Information Solutions, Boulder, Colorado). For global metrics, results were deemed significant at p < 0.05; for nodal metrics, results were deemed significant at False Discovery Rate (FDR) corrected q < 0.05.RESULTS:
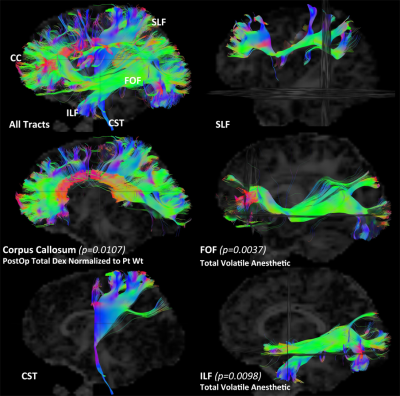
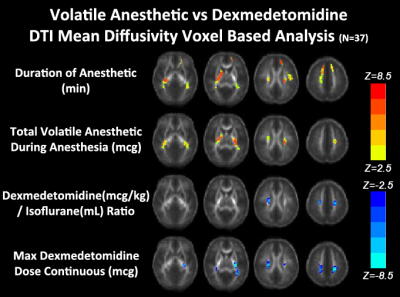
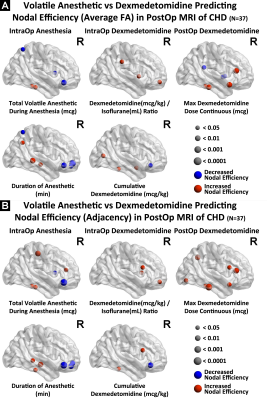
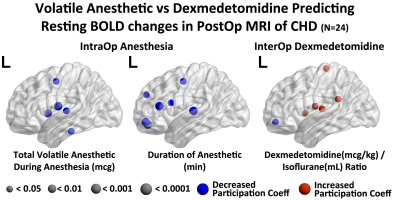
In the hypothesis driven manual tractography analysis, increased total volatile anesthetic dose was associated with reduced structural connectivity involving the FOF (p=0.0037) and the ILF (p=0.0098), with no effect on the CST, CC and SLF (Figure 2). The data driven VBM diffusivity analysis showed that both the duration of anesthetic and total dose of volatile anesthetic were predictive of increased mean diffusivity (opposite of normal developmental change) in frontal lobe regions that overlap with the FOF and ILF, while an opposite effect was noted in relation to DEX (Figure 3).The data driven graph analysis of DTI showed that both duration of anesthetic and total dose of volatile anesthetic was predictive of reduced nodal efficiency in frontal lobe, while an opposite effect was noted in relation to DEX (Figure 4). The data driven graph analysis of resting BOLD showed that both duration of anesthetic and total dose of volatile anesthetic was predictive of reduced brain connectivity (network segregation) in frontal lobe, while an opposite effect was noted in relation to DEX (Figure 5).DISCUSSION & CONCLUSION:
Increased volatile anesthetic exposure in the intraoperative period is associated with reduced post-operative frontal brain connectivity in CHD infants using multiple hypothesis-driven and data-driven analytical approaches. In contrast, intraoperative and postoperative DEX exposure was associated with metrics of improved brain connectivity using the same analytical approach, suggesting in vivo evidence of neuroprotection in CHD infants.Acknowledgements
No acknowledgement found.References
1. Ellen McCann, Mary, and Sulpicio G Soriano. "General anesthetics in pediatric anesthesia: influences on the developing brain." Current drug targets 13, no. 7 (2012): 944-951.
2. Yeh, Fang-Cheng, et al. "Deterministic diffusion fiber tracking improved by quantitative anisotropy." (2013): e80713. PLoS ONE 8(11): e80713. doi:10.1371/journal.pone.0080713
3. Jesper L. R. Andersson and Stamatios N. Sotiropoulos. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage, 125:1063-1078, 2016.
4. Behrens, T. E. J., H. Johansen-Berg, M. W. Woolrich, S. M. Smith, C. A. M. Wheeler-Kingshott, P. A. Boulby, G. J. Barker et al. "Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging." Nature neuroscience 6, no. 7 (2003): 750-757.
5. Friston, Karl J. "Statistical parametric mapping." In Neuroscience databases, pp. 237-250. Springer US, 2003.
6. Rubinov, Mikail, and Olaf Sporns. "Complex network measures of brain connectivity: uses and interpretations." Neuroimage 52, no. 3 (2010): 1059-1069.
Figures