1778
Clinical application of 4D ASL-MRA in neonatal Vein of Galen malformation1Medical Physics and Biomedical Engineering, University College Hospital, London, United Kingdom, 2University College Hospital, London, United Kingdom, 3Leiden University Medical Center, Leiden, Netherlands, 4UCL National Hospital for Neurology & Neurosurgery, London, United Kingdom, 5Great Ormond Street Hospital for Children, London, United Kingdom
Synopsis
This work investigates the feasibility of using time-resolved magnetic resonance angiography, based on arterial–spin-labelling (ASL), to investigate neonatal vein of Galen malformation for the purpose of aiding diagnosis and surgical treatment planning.
Introduction
This work investigates the feasibility of using time-resolved magnetic resonance angiography, based on arterial–spin-labelling (ASL), to investigate neonatal vein of Galen malformation for the purpose of aiding diagnosis and surgical treatment planning. Vein of Galen malformation (VoG) is a rare cerebrovascular malformation (1-2 per 1 million births), in which arterial blood flows directly to the venous circulation via a fistula (arterio-venous shunting). This results in heart failure, impaired blood delivery to brain tissue and untreated is frequently lethal within days. Prognosis is sometimes poor, but early treatment with transarterial embolisation can be life-saving in many patients. Clinical decision-making requires a clear understanding of the pattern and speed of blood through the malformation. The diagnosis is currently inferred from cross-sectional imaging studies (Ultrasound, CT and MRI) but digital subtraction angiography (DSA) is necessary for more detailed analysis. DSA is an invasive, ionising radiation-based technique: contrast is injected via a catheter inserted through the femoral artery into brain arteries under general anaesthetic, yielding 2D projections of vascular flow. Novel MRI techniques provide potential non-invasive substitutes to aid in surgical planning. One such technique, ASL-based time-resolved angiography (4D ASL-MRA) [1,2], was recently shown to be useful for visualising vessel pathologies, such as arterio-venous malformations, as well as aiding planning of gamma-knife radiotherapy [3-5]. It is a non-invasive and non-contrast technique, which makes it an attractive option compared to contrast-based techniques in a neonatal population. This study investigates the feasibility of 4D ASL-MRA in neonates with VoG and explores its added value over standard MR imaging.Methods
Subjects: Three consecutive neonates diagnosed with VoG malformation received an MRI scan on the day of delivery (via caesarean section at GA 37 weeks) as part of their clinical assessment.
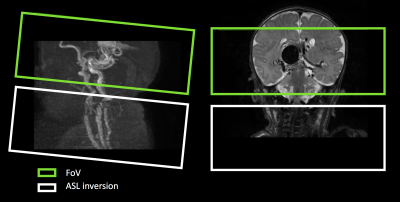
Protocol: Neonates were fitted with hearing protection, placed in an MR-compatible incubator and monitored throughout the MRI session. Scanning was performed on a 3T Philips Achieva scanner (Philips, Best, The Netherlands) using an 8 channel neonatal coil (LMT medical systems). The MRI protocol included: structural T2W, 3DT1W, DWI, ToF and 4D ASL-MRA survey (n=2) and 4D ASL-MRA (3DTFE-EPI TFE factor=7,EPI factor=5,FOV=210x210x60mm,FA=10$$$^\circ$$$,TR=12ms, TE=5ms, axq. matrix: 172x162, 10 timepoints). The temporal resolution of the 4D ASL-MRA was chosen to reflect the rapid flow of blood through VoG based on the 4D ASL-MRA survey scans (TI1=50ms, deltaTI = 115ms). The ASL labelling plane was positioned 20 mm below the field of view (figure1).
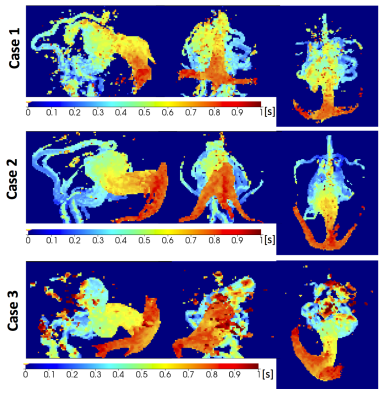
Processing: Maximum intensity projection (MIP) images of the raw ToF images and control-label subtractions of 4D ASL-MRA data were created after masking to exclude extracranial tissue. Additionally, 4D ASL-MRA images were used to create bolus arrival time (BAT) maps, representing the time blood takes to travel from the labelling plane to a given voxel. First, intensities were corrected for $$$T_1$$$ relaxation of blood, the effect of the Look-Locker readout $$$T_{1b,eff} = 1/(1/T_{1b} – log(\cos(FA))/TR)$$$, and the decay of the difference signal dependant on the fraction of time, $$$\beta$$$, that spins experience readout pulses : $$$\exp(-t * (\beta/T_{1b} + (1-\beta)/T_{1b,eff}))$$$ [6], where $$$\beta$$$ was estimated from the data. $$$T1_b$$$ was calculated for each neonate from hematocrit (Hct): $$$ T_{1b} = 0.5*Hct +0.37$$$ [7]. Vessel voxels were identified based on the standard deviation of the signal change with time. Finally, BAT was estimated simply as the time needed to reach 20% of the maximum signal across the interpolated time series.
Results
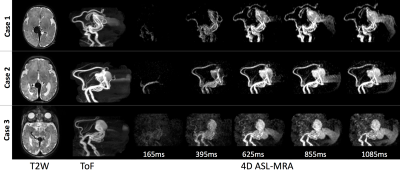
4D ASL-MRA was successfully acquired in all three neonates, despite some motion during acquisition. Figure 2 shows VoG on T2W imaging, sagittal MIP of ToF and every other ASL-MRA timepoint. 4D ASL-MRA images mirror anatomical images well, especially in big vessels. They provide a better contrast in the draining veins compared to ToF. A time resolution of 115ms was sufficient to follow blood progression through the malformation. MIPs of BAT maps (figure 3) provide quantification of blood transit though the vascular shunt and feeding vessels. Additionally, they allow easy comparison between cases for further analysis.Summary & Conclusions
In conclusion, 4D ASL-MRA neonatal VoG provides additional information to the standard structural imaging and aids treatment planning. It offers non-invasive, time-resolved assessment of blood flow through VoG malformation, only previously possible with angiography. 4D ASL-MRA not only provides detailed information about the feeding vessels but also about the rapidity of the shunt ( BAT maps), which may be important for the planning of endovascular treatment and assessemnet of associated risks. It can be safely repeated with different time resolution after inversion pulse or with specific selection of individual vessels for labelling. Future work will compare 4D ASL-MRA with DSA and assess of BAT in treatment outcome.Acknowledgements
This work is supported in part by University College London Hospitals Biomedical Research Centre.References
- Nakamura, M. et al. (2011) Non Contrast Time-Resolved MRA combining High Resolution Multiple Phase EPISTAR (CINEMA-STAR), ISMRM 2011
- Suzuki, Y. et al (2017) Acceleration of ASL-Based Time-Resolved MR Angiography by Acquisition of Control and Labeled Images in the Same Shot (ACTRESS), MRM (in press)
- Jang, J., et al. (2014), 'Non-contrast-enhanced 4D MR angiography with STAR spin labeling and variable flip angle sampling: a feasibility study for the assessment of Dural Arteriovenous Fistula', Neuroradiology, 56 (4), 305-14.
- Fujima N, et al. (2016) ‘Utility of noncontrast-enhanced time- resolved four-dimensional MR angiography with a vessel-selective technique for intracranial arteriovenous malformations.’ J Magn Reson Imaging 44:834–845.
- Rojas Villabona, A, et al. (2017), 'Triple Magnetic Resonance Angiography (Triple-MRA) for Confirmation of Obliteration Following Gamma Knife Radiosurgery for Arterial-Venous Malformations of the Brain', ISMRM2016
- van Osch, M. et al (2005) ‘Non-invasive visualization of collateral blood flow patterns of the circle of Willis by dynamic MR angiography’ Medical Image Analysis 10 (2006) 59–70
- Varela, M. et al (2011) ‘A method for rapid in vivo measurement of blood T1’ NMR in biomedicine, vol. 24, pp. 80–8,
Figures