1776
Anatomo-functional correlates of auditory development in infancy1Cognitive Neuroimaging Unit, INSERM, UMR992; CEA, NeuroSpin Center, Gif-sur-Yvette, France, 2UNATI, CEA DRF Institut Joliot, Gif-sur-Yvette, France
Synopsis
Early infancy is a period of intense behavioral acquisitions and brain development. Nevertheless, how functional and structural maturations are inter-related has been little explored so far. Following studies of visual domain, we aimed to address this question for the auditory modality in 1 to 5-month-old infants, by combining EEG and quantitative MRI measures supposed to reflect fiber myelination and intra-cortical development of dendritic arborization. We investigated the relationships between the functional maturation of auditory-evoked responses in terms of latency and speed, and the maturation of microstructural properties for both white matter tracts and cortical regions of the auditory network.
Introduction
Infant brain development incorporates several mechanisms leading to intense maturation in the neural substrates that occur asynchronously across regions, networks and modalities[1,2]. Yet, how functional and structural changes are inter-related is little understood. Using Electroencephalography (EEG) and diffusion MRI, previous studies of the visual modality showed that the maturation of responses relate to microstructural changes in the corresponding white matter pathways during the first postnatal semester[3,4]. Here we aimed to investigate if such relationships can be established for the auditory modality, and combined EEG and MRI to evaluate the maturation of both white matter tracts and cortical regions.Methods
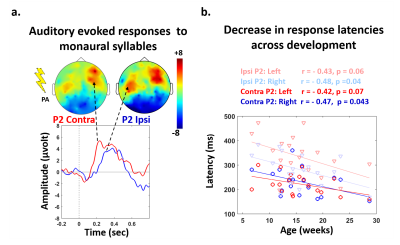
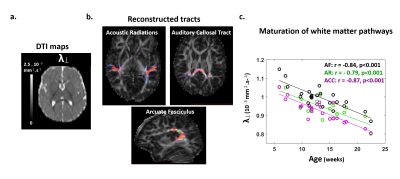
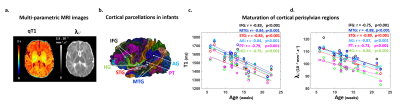
Subjects: 24 healthy infants aged between 6 and 22 weeks were tested with EEG and MRI within a week. Subsets of this initial group had appropriate data for each analysis (see figure legends). Maturation of functional responses: EEG was recorded with a 128-electrode net (EGI®) when infants were listening to syllables presented monaurally with headphones. In each infant, we computed event-related potentials by averaging the recordings from non-artifacted trials. Responses were measured over left and right temporal clusters of electrodes, corresponding to contralateral or ipsilateral hemisphere depending on the side of auditory stimulation. The latency of each P2 response was determined (Figure.1.a), and its speed was estimated by accounting for the brain size. MRI protocol: Acquisitions were performed on a 3T Trio-system (Siemens HealthCare®) with a 32-channel head coil, in <15 minutes during naptime. EPI sequences were used to obtain maps of diffusion tensor imaging (DTI) and longitudinal relaxation time T1[5,6]. Anatomical images were provided by a fast-spin echo sequence[7]. Maturation of white matter tracts: We performed probabilistic tractography based on diffusion images and a 2-crossing-fiber diffusion model[8,9]. In each infant, we identified the left and right acoustic radiations and arcuate fascicles, callosal fibers connecting auditory regions. To characterize the tracts maturation, we quantified DTI transverse diffusivity (λ⟘)[3,4,10]. Maturation of cortical regions: Using accurate intra- and inter-individual co-registrations[11,12], we projected an individual cortical parcellation[7] to each infant’s cortical surface, and measured MRI parameters over auditory-related perisylvian regions. To characterize cortical microstructure, we focused on T1 and DTI longitudinal diffusivity (λ//). Relationships between functional and structural markers of maturation: For each measure, we evaluated age-related dependencies, and asymmetries between left and right hemispheres. To relate the properties of P2 responses and the maturation of white matter tracts or cortical regions, we considered partial correlations accounting for the infants’ age. Statistics were corrected for multiple comparisons.Results
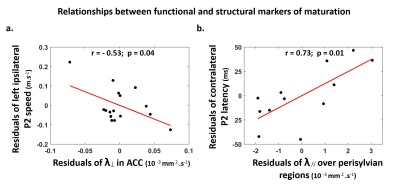
Characterizing functional and structural markers of maturation: Intense age-related changes were observed over the infants group, with decreases in the latency of P2 responses (Figure.1.b), in λ⟘ for all white matter tracts (Figure.2), in T1 and λ// for all cortical regions (Figure.3). We further observed asymmetries notably in the acoustic radiations, arcuate fasciculus, superior and middle temporal gyri (Figure.4). At the functional level, ipsilateral P2 responses had longer latency in the left than right hemisphere, in agreement with a previous study[13] suggesting asymmetric callosal transfer of auditory responses. Relating functional and structural markers of maturation (Figure.5): Only the speed of left ipsilateral P2 responses related to λ⟘ in auditory callosal fibers. Besides, the latency of contralateral P2 responses correlated with λ// over perisylvian cortical regions. For both, increased functional efficiency paralleled microstructural maturation.Discussion
Our study of auditory development in infants highlighted intense maturational changes in all EEG and MRI markers, at the functional and structural levels. Contrarily to the visual modality[3,4], the response speeds were only partly related to the maturation of underlying white matter pathways: this relationship was observed for callosal fibers and left ipsilateral response, which might indeed include a callosal transfer of responses from the right hemisphere[13]. This mismatch between the auditory and visual modalities might rely on differential maturational calendars, with less intense myelination of auditory than visual pathways in the first postnatal months1. In agreement with recentfindings in the aging brain[14], the latency of auditory responses might rather depend on the maturation of cortical regions, as highlighted for contralateral responses. Yet, the microstructural bases for these cortical changes need to be explored over the post-term period, since studies in preterms[15] and animal models[16] put forward the development of dendritic arbors, while children studies[17] highlighted the functional value of microstructural proliferation including intra-cortical myelination.Conclusion
Combining complementary neuroimaging approaches in infants provides the opportunity to characterize the developmental timelines of different functional modalities, which might relate to behavioral acquisitions. In the future, it may also allow to detect early disturbances objectively, and explore altered trajectories in case of neurodevelopmental disorders.Acknowledgements
This research was supported by grants from the Fondation de France and the Fyssen Foundation. The authors would like to thank all the infants and their parents who participated in this study as well as Giovanna Santoro and UNIACTS clinical team for their precious help with data acquisition.References
[1] Yakovlev., & Lecours. (1967). The myelogenetic cycles of regional maturation in the brain.: Oxford: Blackwell.
[2] Dubois, Dehaene-Lambertz, G., Kulikova, S., Poupon, C., Hüppi, P. S., & Hertz-Pannier, L. (2014). The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience, 276, 48-71.
[3] Dubois, Dehaene-Lambertz, G., Soares, C., Cointepas, Y., Le Bihan, D., & Hertz-Pannier, L. (2008). Microstructural correlates of infant functional development: example of the visual pathways. J Neurosci, 28(8), 1943-1948. doi:10.1523/JNEUROSCI.5145-07.2008
[4] Adibpour, Dubois, J., & Dehaene-Lambertz, G. (in press). The right hemisphere, but not the left, discriminates faces in infants. Nature Human Behavior.
[5] Kulikova, S., Hertz-Pannier, L., Dehaene-Lambertz, G., Buzmakov, A., Poupon, C., & Dubois, J. (2014). Multi-parametric evaluation of the white matter maturation. Brain Struct Funct. doi:10.1007/s00429-014-0881-y
[6] Dubois, J., Poupon, C., Thirion, B., Simonnet, H., Kulikova, S., Leroy, F., . . . Dehaene-Lambertz, G. (2016). Exploring the Early Organization and Maturation of Linguistic Pathways in the Human Infant Brain. Cereb Cortex, 26(5), 2283-2298. doi:10.1093/cercor/bhv082
[7] Kabdebon, C., Leroy, F., Simmonet, H., Perrot, M., Dubois, J., & Dehaene-Lambertz, G. (2014). Anatomical correlations of the international 10-20 sensor placement system in infants. Neuroimage, 99, 342-356. doi:10.1016/j.neuroimage.2014.05.04
[8] Behrens, T. E., Berg, H. J., Jbabdi, S., Rushworth, M. F., & Woolrich, M. W. (2007). Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage, 34(1), 144-155. doi:10.1016/j.neuroimage.2006.09.018
[9] FSL : http://fsl.fmrib.ox.ac.uk/fsl
[10] Hua, K., Zhang, J., Wakana, S., Jiang, H., Li, X., Reich, D. S., . . . Mori, S. (2008). Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage, 39(1), 336-347. doi:10.1016/j.neuroimage.2007.07.053
[11] Lebenberg, J., Poupon, C., Thirion, B., Leroy, F., Mangin, J.-F., Dehaene-Lambertz, G., Dubois, J., 2015. Clustering the infant brain tissues based on microstructural properties and maturation assessment using multi-parametric MRI, in: 2015 IEEE 12th International Symposium on Biomedical Imaging (ISBI). pp. 148–151. doi:10.1109/ISBI.2015.7163837
[12] Lebenberg, Mangin, Thirion, Poupon, Hertz-Pannier, Leroy, Adibpour, Dehaene-Lambertz, Dubois. (in prep). Mapping the asynchrony of cortical maturation with MRI:a multi-parametric clustering approach in the infant brain.
[13] Adibpour, Dubois, Moutard, & Dehaene-Lambertz. (under-review). Early asymmetric inter-hemispheric transfer in the auditory network: insights from infants with corpus callosum agenesis.
[14] Price, D., Tyler, L., Henriques, R. N., Campbell, K., Williams, N., Treder, M., & Taylor, J. (2017). Age-related delay in visual and auditory evoked responses is mediated by white-and grey-matter differences. Nature Communications, 8.
[15] Ball, G., Srinivasan, L., Aljabar, P., Counsell, S. J., Durighel, G., Hajnal, J. V., . . . Edwards, A. D. (2013). Development of cortical microstructure in the preterm human brain. Proceedings of the National Academy of Sciences, 110(23), 9541-9546.
[16] Wang, X., et al., Folding, but not surface area expansion is associated with cellular morphological maturation in the fetal cerebral cortex. J Neurosci, 2017
[17] Gomez, J., Barnett, M. A., Natu, V., Mezer, A., Palomero-Gallagher, N., Weiner, K. S., . . . Grill-Spector, K. (2017). Microstructural proliferation in human cortex is coupled with the development of face processing. Science, 355(6320), 68-71.
Figures