1774
Asynchrony of the cortical maturation in the infant brain studied with MRI1UNATI, CEA DRF/Institut Joliot, Université Paris-Sud, Université Paris-Saclay, NeuroSpin center, Gif-sur-Yvette, France, 2Cognitive Neuroimaging Unit U992, INSERM, CEA DRF/Institut Joliot, Université Paris-Sud, Université Paris-Saclay, NeuroSpin center, Gif-sur-Yvette, France, 3Multicenter Neuroimaging Platform, CATI, cati-neuroimaging.com, France, 4UNIRS, CEA DRF/Institut Joliot, Université Paris-Sud, Université Paris-Saclay, NeuroSpin center, Gif-sur-Yvette, France, 5UNIACT, CEA DRF/Institut Joliot, INSERM U1129, Université Paris-Sud, Université Paris-Saclay, Université Paris-Descartes, NeuroSpin center, Gif-sur-Yvette, France
Synopsis
Intense changes in cortical microstructure occur during early infancy. Here, we aimed to study cortical maturation over this largely unexplored developmental period using quantitative MRI in 17 infants from 1 to 5 post-natal months. By taking benefit of robust intra- and inter-individual registrations of anatomical images and parametric maps, we measured T1, T2 relaxation times, and DTI longitudinal diffusivity over cortical surfaces and regions of interest. Results showed that each parameter relevantly but differently reflects the progressive maturation. This suggests that multi-parametric approaches might provide interpretable measures of the developing microstructure by accounting for the parameters complementarity.
Introduction
Intense changes in cortical microstructure occur after birth with the development of dendritic arborization, synaptogenesis and fiber myelination1,2. These maturational processes are supposed to relate to massive psycho-motor acquisitions, but direct evidence is still lacking in infants. Here, we aimed to quantify the cortical maturation with MRI over a largely unexplored developmental period. We focused on different quantitative parameters (longitudinal T1 and transverse T2 relaxation times, longitudinal diffusivity λ// from diffusion tensor imaging DTI)3,4 which were mapped to individual cortical ribbons extracted from anatomical images. These parameters were further compared according to a region-based analysis using inter-subject registration and a cortical parcellation5.Material and methods
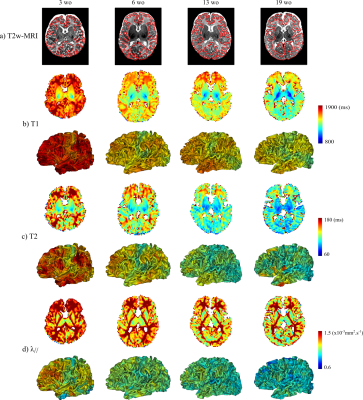
The study was conducted in 17 healthy term-born infants (age at MRI: 3-21 weeks), under a protocol approved by the Institutional Ethical. Acquisitions were performed on a 3T Trio Siemens system. Echo-planar imaging (EPI) sequences were used to estimate T1, T2 and DTI maps in less than 11min (1.8mm isotropic spatial resolution)3,6. T2-weighted images (1x1x1.1mm3) were acquired with a turbo-spin echo sequence5, and processed semi-automatically to reconstruct inner cortical surfaces and extract cortical sulci7,8. Parametric maps were registered to T2-weighted images using elastic deformations4 providing an accurate overlay of cortical ribbons across modalities. T1, T2 and λ// values were then projected on cortical surfaces (Figure 1).
To align cortical surfaces across infants, we used a 2-step registration strategy. The DISCO approach9 aimed to align selected cortical sulci (easily recognizable in infants and covering the whole brain (see Figure 2) , while the DARTEL algorithm10 aimed to register cortical strips over the group.
Based on this DISCO+DARTEL registration, we computed average T1, T2 and λ// maps over the group, and we analyzed these parameters over cortical regions using an infant parcellation manually delineated from one of the studied subject5 and deformed by the DISCO+DARTEL deformations. We here selected some regions of interest to highlight differences in maturation and assess the parameters significance and complementarity.
Results
Compared with 1-step strategies, the DISCO+DARTEL approach improved the registration of anatomical images, cortical surfaces, sulci and strips over the group (Figure 2).
Across infants, we observed that T1, T2 and λ// decreased with age, and were higher in frontal, lateral occipital and temporal regions than in primary cortices (Figure 1). Nevertheless, these maturational differences were not always obvious at the individual level, depending on the infant and parametric map.
This asynchrony was better revealed on average maps over the group (Figure 3), with lower T1, T2 and λ// in primary than associative regions. Nevertheless, these parameters showed differences in terms of value distributions over the whole brain, and of maturational ordering across the selected cortical regions (especially for Heschl and inferior temporal gyri).
Discussion
In this study, we reliably evaluated the maturation of cortical microstructure in infants, over a period that has been little studied so far. In comparison with previous studies of the developing brain2,11,12, the use of quantitative MRI parameters stemming from relaxometry and diffusometry enabled us to compare regions and subjects without requiring additional processing (e.g. spatial bias correction, signal normalization). Nevertheless, with our approach, the differential maturation across cortical regions was much more visible over the group than at the individual level.
Generally, we observed a more advanced maturation in primary regions than in adjacent unimodal and higher-order associative regions, in agreement with benchmark post mortem studies of cortical synaptogenesis1 and sub-cortical white matter myelination13. Contrarily to recent descriptions in infants from one year of age14, we did not observe relationships between cortical maturation, surface curvature or folding patterns (except around the central sulcus and calcarine fissure that house primary sensori-motor and visual cortices).
Finally, it seems that T1, T2 and λ// parameters provide distinct information on the maturation and microstructure of the infant cortical regions. A multi-parametric approach may thus take benefit from this complementarity as proposed for white matter bundles3.
Conclusion
Characterizing the asynchronous maturation of cortical regions in infants with MRI is a promising approach on several issues. In parallel to microstructural changes, the cortex demonstrates intense morphological evolution and growth throughout infancy. Since the most recently developed regions along the primate lineage also show the highest surface expansion during human infancy15, it would be important to investigate whether developmental changes at the microstructural and morphological levels are correlated. It might also give the opportunity to explore the relationships between cortical maturation and the development of functional systems and behavioral capacities throughout infancy. Along these lines, recent studies in children suggested microstructural bases of cognitive performances such as face recognition16.Acknowledgements
This project has received funding from the European Union's Horizon 2020 Framework Program for Research and Innovation under Grant Agreements No 720270 (Human Brain Project SGA1) and No. 604102 (HBP’s ramp-up phase). Infant MRI acquisitions were financed thanks to grants from the Fondation de France and Fyssen Foundation. The authors thank the UNIACT clinical team from NeuroSpin for precious help in scanning and segmenting infants’ images as well as Thierry Delzescaux for his advices and tools about the intra-subject registration.References
1. Huttenlocher, P.R., Dabholkar, A.S.. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997; 387, 167–178.
2. Travis, K.E., Curran, M.M., Torres, C., Leonard, M.K., Brown, T.T., Dale, A.M., Elman, J.L., Halgren, E.. Age-related Changes in Tissue Signal Properties Within Cortical Areas Important for Word Understanding in 12- to 19-Month-Old Infants. Cereb. Cortex N. Y. NY. 2014; 24, 1948–1955.
3. Kulikova, S., Hertz-Pannier, L., Dehaene-Lambertz, G., Buzmakov, A., Poupon, C., Dubois, J.. Multi-parametric evaluation of the white matter maturation. Brain Struct. Funct. 2015 ; 220, 3657–3672.
4. Lebenberg, J., Poupon, C., Thirion, B., Leroy, F., Mangin, J.-F., Dehaene-Lambertz, G., Dubois, J.. Clustering the infant brain tissues based on microstructural properties and maturation assessment using multi-parametric MRI, in: IEEE 12th International Symposium on Biomedical Imaging (ISBI). 2015, pp. 148–151.
5. Kabdebon, C., Leroy, F., Simmonet, H., Perrot, M., Dubois, J., Dehaene-Lambertz, G.. Anatomical correlations of the international 10-20 sensor placement system in infants. NeuroImage. 2014; 99, 342–356.
6. Dubois, J., Poupon, C., Thirion, B., Simonnet, H., Kulikova, S., Leroy, F., Hertz-Pannier, L., Dehaene-Lambertz, G.. Exploring the Early Organization and Maturation of Linguistic Pathways in the Human Infant Brain. Cereb. Cortex N. Y. NY. 2016 ; 26, 2283–2298.
7. Fischer, C., Operto, G., Laguitton, S., Perrot, M., Denghien, I., Rivière, D., Mangin, J.-F.. Morphologist 2012: the new morphological pipeline of BrainVISA. Presented at the OHBM, 2012.
8. Leroy, F., Mangin, J.-F., Rousseau, F., Glasel, H., Hertz-Pannier, L., Dubois, J., Dehaene-Lambertz, G.. Atlas-Free Surface Reconstruction of the Cortical Grey-White Interface in Infants. PLoS ONE. 2011 ; 6, e27128.
9. Auzias, G., Colliot, O., Glaunes, J.-A., Perrot, M., Mangin, J.-F., Trouve, A., Baillet, S.. Diffeomorphic Brain Registration Under Exhaustive Sulcal Constraints. IEEE Trans. Med. Imaging. 2011 ; 30, 1214–1227.
10. Ashburner, J.. A fast diffeomorphic image registration algorithm. NeuroImage. 2007; 38, 95–113.
11. Leroy, F., Glasel, H., Dubois, J., Hertz-Pannier, L., Thirion, B., Mangin, J.-F., Dehaene-Lambertz, G.. Early maturation of the linguistic dorsal pathway in human infants. J. Neurosci. Off. J. Soc. Neurosci. 2011; 31, 1500–1506.
12. Grydeland, H., Walhovd, K.B., Tamnes, C.K., Westlye, L.T., Fjell, A.M.. Intracortical Myelin Links with Performance Variability across the Human Lifespan: Results from T1- and T2-Weighted MRI Myelin Mapping and Diffusion Tensor Imaging. J. Neurosci. 2013; 33, 18618–18630.
13. Flechsig, P.. Anatomie des menschlichen Gehirns und Rückenmarks. 1920.
14. Deoni, S.C.L., Dean III, D.C., Remer, J., Dirks, H., O’Muircheartaigh, J.. Cortical maturation and myelination in healthy toddlers and young children. NeuroImage. 2015; 115, 147–161.
15. Hill, J., Inder, T., Neil, J., Dierker, D., Harwell, J., Essen, D.V.. Similar patterns of cortical expansion during human development and evolution. Proc. Natl. Acad. Sci. 2010; 201001229.
16. Gomez, J., Barnett, M.A., Natu, V., Mezer, A., Palomero-Gallagher, N., Weiner, K.S., Amunts, K., Zilles, K., Grill-Spector, K.. Microstructural proliferation in human cortex is coupled with the development of face processing. Science. 2017 ; 355, 68–71.
Figures