1767
Preeclampsia related to delayed development of white matter and cortical infolding.Ting Liu1, Miaomiao Wang1, Chao Jin1, Xianjun Li1, and Jian Yang1
1Department of Diagnostic Radiology, the first Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
Synopsis
Offspring born from preeclampsia exhibit deficits in cognitive impairment. But the pathogenesis is not clear. We assessed brain maturation and white matter development in neonatal period using total maturation score and tract-based spatial statistics. TMS showed the scores of TMS, B and C scores were lower in preeclampsia group. TBSS results displayed FA values decreased, while AD and RD values increased on anterior & posterior limb of internal capsule, external capsule, splenium of corpus callosum, optic radiation and centrum semiovale in preeclampsia group. The results indicated preeclampsia is associated with delayed development of white matter and cortical infolding.
Introduction
Offspring born from preeclampsia pregnancies (PE-F1s) exhibit deficits in working memory, visuospatial processing and lower intelligence quotient scores1-3. But the pathogenesis is still not very clear. Little is known about brain changes in neonatal period, although some evidence displays differences of brain anatomy and function between PE-F1s and children born from normotensive mothers4,6. In this study, we have assessed brain maturation and white matter injury in neonatal period by using total maturation score (TMS) and tract-based spatial statistics (TBSS) respectively. These result showed that the delayed brain development of PE-F1s can be distinguished in neonatal period.Materials and Methods
This study was approved by ethical committee of the First Affiliated Hospital. Written parental informed consent was obtained prior to the scan for all neonates. 71 neonates underwent brain MRI within 28 days after birth were enrolle, including 19 infants from preeclampsia (mean PMA: 45.44±5.10 weeks) and 52 control infants whose mothers with normal blood pressure (mean PMA: 46.71±5.78 weeks). Brain MR examinations for each newborn was performed on a 3T MRI system (GE Healthcare, Milwaukee, Wisconsin) with an 8-channel head coil. Each neonatus were sedated during imaging using oral chloral hydrate (25-50 mg/kg) with parental consent. (1) Diffusion tensor imaging (DTI) was acquired in 35 diffusion directions with the following parameters: TR 5500 ms; TE 95 ms, section-thickness 4mm without gap, FOV 180mm, matrix 128×128, b value 1000s/mm2, SENSE factor 2. (2) T1WI 3D magnetization prepared rapid gradient echo (3D-MPRAGE) images were acquired using: TR 10 ms; TE 4.6 ms, FOV 240mm, matrix 128×128, section-thickness 1mm. (3) T2WI fast spin echo (FSE) images were acquired using: TR 4200 ms; TE 120 ms, FOV 180mm, matrix 256×256, section-thickness 4mm. DTI data were processed using TBSS by FMRIB (FSL, www.fmrib.ox.au.uk/fsl). Two pediatric radiologists calculated the TMS, including the four parameters of TMS scores--progressive myelination (M), cortical infolding (C), involution of germinal matrix tissue (G), and glial cell migration bands (B), independently. The mean values of the scores was used for statistical analysis. All statistical analysis were performed by using SPSS 18.0 software (SPSS, Chicago, IL, USA); p<0.01 was considered significant difference.Results
The cohort characteristics of two groups was seen in Table 1. There was no significant difference in PMA, sex and Apgar score at 10 minutes between cohorts. However, the weight at birth of preeclampsia group was significantly lower than the control cohor. Brain maturation analysis showed that the scores of TMS (11.00 ± 1.17) were significantly lower in preeclampsia group than it in the control group (11.96 ± 1.41, p<0.01). The parameters of TMS, the M (2.59 ± 0.61 vs. 2.93 ± 0.40, p<0.05) and C scores (3.47 ± 0.67 vs. 3.91 ± 0.81, p<0.01) were also lower in preeclampsia group (Figure 1). TBSS based on DTI displayed that FA values decreased, while AD, and RD values increased significantly on anterior & posterior limb of internal capsule, external capsule,splenium of corpus callosum, optic radiation and centrum semiovale (Figure 2).Discussion
This study indicated that infants from preeclampsia displayed poor progressive myelination and cortical infolding compared infants from normotensive mothers. This suggest that infants from preeclampsia showing signs of a delay on brain development. TBSS-analyse showed difference of FA, AD and RD in anterior & posterior limb of internal capsule, external capsule, splenium of corpus callosum, optic radiation and centrum semiovale of two group. That implying that these region of white matter was dysplasia, and the function of the white matter area also can be impaired. Previous research has shown that PE-F1s showed greater cognitive decline, such as working memory and visuospatial processing, and lower IQ scores5. The reason must be found on the brain. It was reported that PE-F1s showed higher FA values in the caudate nucleus, which is an analysis for child aged 7-10. That is different from our study, because the object of our study is neonates and many factors of environment could impact the brain development when the infant grows up. It is also displayed that PE-F1s showed enlargement regional volumes in cerebellum, temporal lobe, brainstem and right and left amygdala6. These proofs reveal the abnormal of brain development of PE-F1s.Conclusion
Preeclampsia is associated with delayed development of white matter and cortical infolding.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFC0100300), National Natural Science Foundation of China (No. 81471631, 81771810 and 51706178), the 2011 New Century Excellent Talent Support Plan of the Ministry of Education, China (NCET-11-0438) the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University (No.XJTU1AF-CRF-2015-004).References
1.Frances D, B. Anne C, Patrick W. S, et al. Impacts of Preeclampsia on the Brain of the Offspring. Rev Bras Ginecol Obstet, 2016, 38:416-422. 2. Matthew T. R, Andrew F. H, Brandon M, et al. Impact of preeclampsia on cognitive function in the offspring. Behavioural Brain Research, 2016, 302:175-81. 3. Many A, Fattal A, Leitner Y, et al. Neurodevelopmental and cognitive assessment of children born growth restricted to mothers with and without preeclampsia. Hypertension in pregnancy. 2003, 22:25-9. 4. Ernesto A. F, B. Anne C, Reynolds JN et al. Tensor Imaging of White Matter in Children Born from Preeclamptic Gestations. AJNR American journal of neuroradiology, 2017, 38:801-806. 5. Morsing E, Maršál K. Pre-eclampsia- an additional risk factor for cognitive impairment at school age after intrauterine growth restriction and very preterm birth, Early Hum Dev, 2014, 90: 99–101. 6. Rätsep MT, Paolozza A, Hickman AF, et al. Brain structural and vascular anatomy is altered in offspring of pre-eclamptic pregnancies: a pilot study. AJNR Am J Neuroradiol, 2016, 37:939-945.Figures
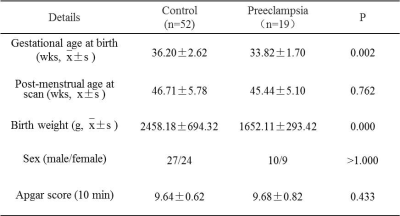
Table 1 The cohort characteristics
of the preeclampsia and control group.
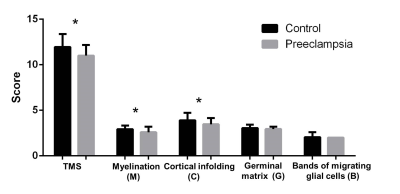
Figure 1 Analysis of the total maturation score (TMS), including score of total maturation score (TMS), progressive myelination
(M), cortical infolding (C), involution of germinal matrix tissue (G) and glial cell migration
bands (B) for preeclampsia and control group.*P<0.01.
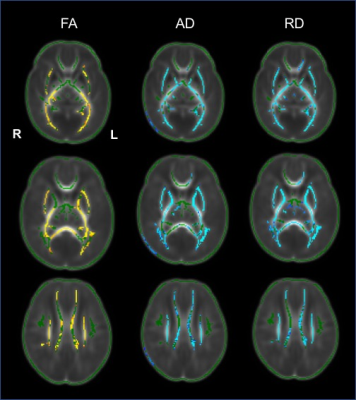
Figure 2 Tract-based spatial
statistics for comparisons between the preeclampsia group and control groups. Regions
showed yellow-red represent decreased voxels significantly (P < 0.05), and light
blue-blue represent increased voxels significantly (P < 0.05) in the in preeclampsia
group compared the control group.