1764
Gradient system characterization of a 1.5T MRI-Linac with application to UTE imaging1Radiotherapy, University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
We characterize the gradient system of a hybrid 1.5T MRI-Linac, which has been developed as the ideal platform for MRI-guided radiotherapy. The system is equipped with a split gradient coil that potentially complicates reconstruction of non-Cartesian sequences such as ultra short echo time (UTE) imaging, which is a promising sequence for pseudo-CT generation and lung imaging. Here, we determine the zeroth and first spatial order gradient impulse responses. These are used to show that UTE imaging is feasible and image quality can be increased significantly using the gradient impulse response.
Purpose:
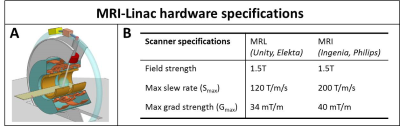
Simultaneous MRI and external beam radiotherapy will enable on-line organ contour adaptation based on the current anatomy and is therefore a promising way to achieve highly localized radiotherapy treatments. Although the 1.5T MRI-Linac (MRL), developed at our institute (Unity, Elekta, Crawley, UK), is based on a conventional diagnostic Philips Ingenia system, design modification were required to accommodate the passage of the therapeutic photon beam[1]. Most importantly, this required a 200 mm gapped split gradient coil design (Fig.1-A), which has implications on gradient performance and eddy current behavior. In this work we characterize the zeroth and first spatial order gradient impulse response functions (GIRFs) of the MRL versus a 1.5T diagnostic system, including the employed pre-emphasis filters, and demonstrate its impact on the image quality of ultra short echo time (UTE) reconstructions.Methods
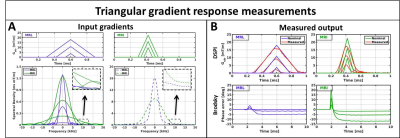
Gradient system characterization: Measurements were performed on a 1.5T Philips Ingenia diagnostic system and on a 1.5T MRL (Elekta Unity) system with hardware specifications summarized in Fig.1-B. Twenty-one triangular gradients with varying amplitudes and maximum slew rate were measured on a 15 cm diameter spherical phantom (Fig.2-A). The zeroth and first order responses of the triangular gradients were measured using the Brodsky method[2] and dynamic Single Point Imaging (DSPI)[3], respectively. The system was assumed as linear time-invariant to determine zeroth and first order GIRFs[4]. Relevant parameters for DSPI: 401 single point imaging encodings, TR=5 ms, sampling frequency=600 kHz, readout duration=2 ms, scan time=45 s per axis. Relevant parameters for Brodsky: TR=30ms, sampling frequency=400 kHz, readout duration=20 ms, 100 averages, scan time=6 min per axis.
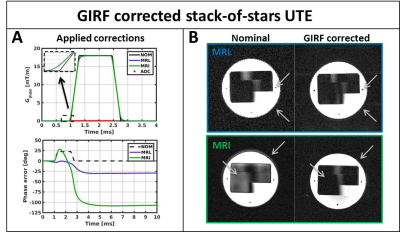
UTE imaging experiments: Phantom and in-vivo imaging data were acquired to investigate the impact of GIRF-corrected reconstructions on the image quality of UTE acquisitions. Phantom data were acquired on both systems using a 3D RF- and gradient-spoiled gradient echo with stack-of-stars ultrashort echo time (UTE) readout. To allow a fair comparison between the systems the hardware settings were constrained to the clinically used settings for the MRL (Smax=65 T/m/s and Gmax=15 mT/m). Free-breathing abdominal MRL data were acquired using golden angle stack-of-stars trajectory with the first echo as UTE readout. Image reconstruction was performed in two steps: 1) K-space data were phase corrected using the zeroth order GIRF: 2) K-space trajectory was corrected using the first order GIRF followed by density compensation and a 3D non-uniform fast Fourier transform[5].
Results and discussion:
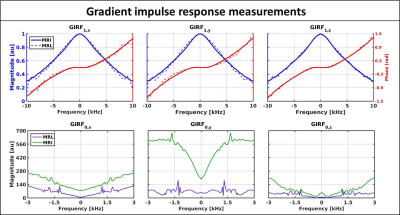
The measured responses of the triangular gradients are shown in Fig.2-B, zeroth order effects were measured as phase accumulations and first order as gradient waveform errors. The measured GIRFs are shown in Fig.3 for both systems and are displayed according to their spatial order and gradient axis(GIRForder,axis). The GIRF1,z of both systems is very similar and the GIRFs1,xy of the MRL show slightly better gradient fidelity around the lower frequencies. The GIRF0,y has considerably larger magnitudes than the other axes on the diagnostic system, while this discrepancy completely vanishes on the MRL. This finding seems to contradict the initial hypothesis that the split gradient coil design would be more susceptible to eddy currents. However, the MRL system operates with a newer generation pre-emphasis filter that could explain some of the observed differences. The GIRFs0,xyz show distinct peaks at≈1800 Hz for both systems and an additional≈1200 Hz peak for the MRL, which are presumably mechanical resonances.
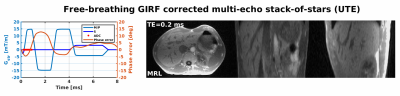
The effect of the GIRF-corrected image reconstruction for a single echo stack-of-stars UTE phantom acquisition is shown in Fig.4. The typical halo artefacts are reduced, edges sharpen up and overall image quality is considerably improved. Fig.5 shows GIRF-corrected reconstructions of abdominal in vivo multi-echo UTE MRL data that considerably improved image quality compared to the conventional case(not shown). While the overall image quality of the abdominal data is exquisite, some residual shading artefacts are present in the second and third echo. These artefacts may indicate higher order field imperfections, however the characterization of these effects remains future work.
Conclusion:
Zeroth and first order GIRFs were characterized on a 1.5T MRL system and a 1.5T diagnostic MR system. GIRF-corrected image reconstructions considerably improved image quality for UTE acquisitions on both systems. We hypothesize that GIRF-corrected image reconstructions are highly beneficial for non-Cartesian acquisitions, such as spirals, which we believe will be invaluable for MRI-guided radiotherapy on the MRL.Acknowledgements
This work is part of the research programme HTSM with project number 15354, which is (partly) financed by the Netherlands Organisation for Scientific Research (NWO).References
[1] Lagendijk J, Raaymakers B, Raaijmakers A, et al. MRI/linac integration. Radiother. Oncol. 2008;86:25–29.
[2] Brodsky E, Klaers J, Samsonov A, Kijowski P, et al. Rapid Measurement and Correction of Phase Errors from B0 Eddy Currents: Impact on Image Quality for Non-Cartesian Imaging. Magn. Reson. Med. 2013;69(2): 509–515.
[3] Jang H, Mcmillan A. A Rapid and Robust Gradient Measurement Technique Using Dynamic Single-Point Imaging. Magn. Reson. Med. 2016;78;950-962
[4] Vannesjo S, Haeberlin M, Kasper L, et al. Gradient system characterization by impulse response measurements with a dynamic field camera. Magn. Reson. Med. 2013;69:583–593.
[5] Fessler J and Sutton B, Nonuniform fast fourier transforms using min-max interpolation, IEEE Trans. Signal Process., vol. 51, no. 2, pp. 560-574, Feb. 2003.
Figures