1749
Development of an integrated RF coil and restraint system for awake rat scanning at 7T1McGill University/Douglas Hospital, Montreal, QC, Canada
Synopsis
Research utilizing awake rodents has been conducted for the past 10-15 years, however limitations still exist surrounding this technique. Our goal is to build a restraining/RF coil system that circumvents some of the shortcomings present in existing systems, while allowing for the delivery of various stimuli during preclinical neuroimaging. The proposed design (i.e. TriCoil) has integrated access ports for binocular visual stimulation, gustatory and olfactory stimuli presentation, as well as intranasal delivery. SNR obtained with the TriCoil was superior to a volumetric RF coil for awake rat imaging, while a CO2 challenge yielded significant brain-wide BOLD changes.
Purpose
Exponential progress in pre-clinical research has been made with the development of imaging hardware designed for small animals (predominantly mice and rats). Although preclinical imaging studies offer valuable insight into brain-wide activity, the results are often confounded by use of anesthetic agents1. These agents have been shown to directly affect neural/glial activity, CBF, CBV and ultimately BOLD responses. This in turn poses serious issues with regards to the clinical translatability of findings yielded from these studies. In addition, anesthetized preclinical imaging does not allow for task-based fMRI experimental design. To this end, this work describes the development of an integrated RF coil and restraint system, allowing for awake rat neuroimaging at 7T. The design consists of a 3-element RF array that enables multimodal stimulation (i.e. visual, gustatory, intracranial etc.) during the scan in awake rats, with improved basal coverage relative to single loop coil designs.Methods
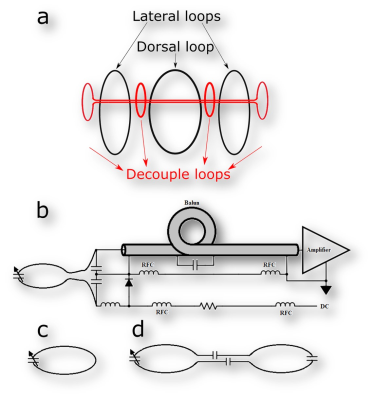
Non-invasive restraining mechanism. The TriCoil housing was designed using the Sketchup software, and printed in ABS plastic. The system has an integrated head restraint system with panels that press laterally (lightly) on the animal’s head to minimize head motion. One RF loop is fitted into each one of the lateral panels, while the third loop is fitted and on top of the rat’s head, which is integrated into the dorsal restraint panel. The head restraint system is further fitted with small lateral openings allowing for the passage of optic fibers necessary for binocular visual stimulation, and six additional openings are built into the head-holder, immediately anterior to the rat’s snout, allowing for access to the nostrils via small caliber PE tubing (2 openings), and the mouth for gustatory stimulation (2 openings). Two additional openings are designed to allow for olfactory presentation. Having two openings per sensory domain allows for different stimuli to be presented during one scanning session. RF coil system. Preamplifier decoupling is employed utilizing low-impedance Siemens preamplifiers (Fig. 1a). Since the coil elements are housed within each independently adjustable restraining arm, the loops have no geometric overlap. As a result, preamplifier decoupling alone was not sufficient to minimize coupling; additional passive decoupling loops was adjusted with variable capacitors which were placed between adjacent loops. The capacitor was adjusted to further minimize coupling between elements (Fig. 1b). The dorsal loop and its housing were constructed with a central opening to allow the passage of intracranial implants during MRI, if necessary2. To avoid potential effects of anesthetic agents on brain function, rats were scanned awake, following acclimation sessions (1/day for 7 days). Scans were conducted on a Bruker 70/30 MRI system. A gradient echo based fast low angle shot (FLASH) sequence (TR/TE = 350/5.4 ms, repetitions = 3, flip angle = 40◦, slices = 23, slice thickness = 1.2 mm, spatial resolution = 0.15 mm x 0.15 mm, matrix size = 256 × 256, acquisition time = 3 m 21s) was used for SNR quantification. SNR performance of the TriCoil was compared against a commercially available rat brain optimized 45-mm volumetric coil (Animal Imaging Research, Boston, MA). Functional images were acquired using an EPI pulse sequence (TR/TE = 900/15 ms, slice thickness = 1.2 mm, matrix size = 512 × 512, acquisition time = 9 min) during a CO2 challenge (3 min baseline, followed by 3 min CO2 exposure, then 3 min washout).Results
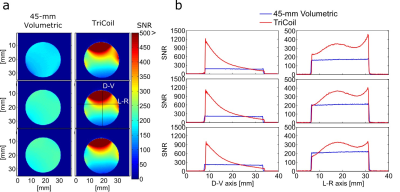
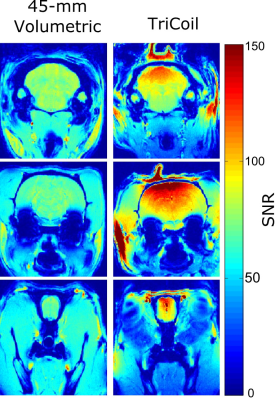
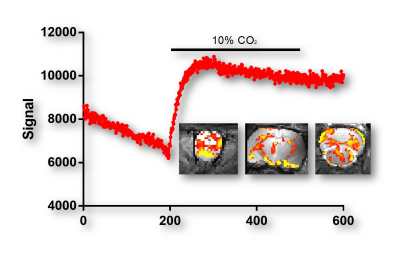
Decoupling loops reduced coupling between elements to -18dB or less. SNR comparisons between the 45-mm volumetric coil and TriCoil in phantom are illustrated in Fig. 2, and in a rat brain preparation Fig. 3. SNR of the TriCoil is seen to be highest proximal to the dorsal loop. with minimized SNR drop-off along the dorsoventral (D-V) axis was mitigated by the presence of the lateral elements. The CO2 challenge resulted in distributed positive BOLD signal change, as shown in Fig. 4.Conclusion
The current design represents a novel approach to awake animal imaging, in which an array RF is integrated with awake restraint and stimuli delivery systems. Future work will demonstrate the use of this system to acquire functional MRI data in awake rats under various types of stimuli.Acknowledgements
This work has been supported by NSERC (Grant No. RGPIN-2014-07072) awarded to JN.References
1. Madularu, D., et al. A non-invasive restraining system for awake mouse imaging. Journal of neuroscience methods 287, 53-57 (2017).
2. Madularu, D., et al. A chronic in situ coil system adapted for intracerebral stimulation during MRI in rats. Journal of neuroscience methods 284, 85-95 (2017).
Figures