1748
Investigating the Coverage of Receive Coil Arrays Through the SNR and Parallel Imaging Performance: A Simulation Study on A Realistic Monkey Head Model at 7T1Interdisciplinary Institute of Neuroscience and Technology, Qiushi Academy for Advanced Studies, Zhejiang University, Hangzhou, China, 2College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, China, 3Key Laboratory for Biomedical Engineering of Ministry of Education, Zhejiang University, Hangzhou, China
Synopsis
The coverage of receive coil array is an important concern in coil design especially for monkey head coil. The simulation of receive coil array is helpful in decision-making. For macaque brain imaging at 7T, five coil array configurations with different coil coverage under realistic considerations were systematically evaluated through quantifying their spatial SNR profiles and parallel imaging acceleration performance. Extending the traditional helmet coverage design for monkey head to whole-head coverage demonstrated substantial improvement in acceleration performance in deep brain region, but less pronounced enhancement can be observed in spatial SNR profiles in brain area.
Introduction
Non-human primates are a valuable animal model in neuroscience research. To image cortical functional columns and laminar neural activity, sub-millimeter resolution and high temporal resolution are both required.1,2 Using a multi-channel receive array with the capability of acquisition acceleration is necessary and vital in meeting this goal; moreover, a shorter echo spacing towards less susceptibility effect and a smaller echo time for better BOLD contrast can be attained with parallel imaging acceleration.3 A variety of monkey head coils have been developed to assist functional MR study. Because of the demand for head-fixation and physiological intervention, monkey head coils usually have less coverage than those for human head.3,4 As a consequence, deep brain region and cortical area near face and shoulders may suffer from SNR deterioration and higher SNR penalty in imaging acceleration. In this study, we try to find a good tradeoff between limited coil coverage vs. the SNR and parallel imaging performance through simulating various scenarios of coil coverage configurations using a full wave approach.5Methods
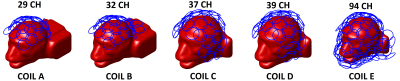
Five different coil configurations consisting of overlapped loop elements on a conformal surface under realistic constraint in space were constructed (Fig.1): coil A) 29 channels, leaving space for ear-bar, bite-bar and visual stimulus; coil B) 32 channels, similar to coil A, but with extra coverage around shoulder; coil C) 37 channels, leaving space for head-post, bite-bar and visual stimulus; coil D) 39 channels, similar to coil C but with two more loops to cover the top of head and leave no space for head-post; coil E) 94 channels, leaving no space for head-fixation or visual stimulus. Coil geometry of each array was generated automatically following the hexagon pattern under predefined coil coverage constraint. Every loop element shared the same diameter (3 cm) and the same distance (1 cm) above the monkey head surface. We used the fast EM solver MARIE6,7 to simulate SNR and parallel acceleration capabilities for each coil. The monkey head model with 2mm isotropic resolution was constructed from MPRAGE images over a macaque head8. The electric properties of each tissue were assigned with the values of human’s at 300 MHz. To simulate the effect of putting extra coverage around shoulder properly, the macaque model mounted within coil A and B was extended behind shoulder to mimic the C-spine and shoulder areas. Each loop element has two ports and was tuned/matched in circuit co-simulation. The inductance coupling was ignored and the noise co-variance matrix was extruded from g-factor calculation to mimic the effect of preamplifier decoupling. The spatial SNR of each coil array was calculated using the optimized combination method in SENSE reconstruction9. G-factors were calculated using in-plane acceleration factor of RSENSE = 3 at directions of F-H, A-P, and L-R, respectively, by assuming the macaque was placed inside a horizontal MRI bore in the prone position.Results
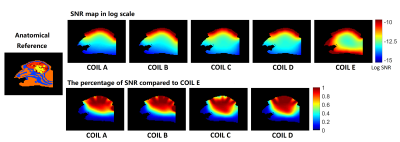
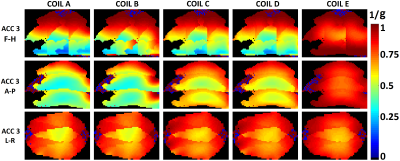
For all coil configurations, very similar spatial SNR profiles are observed within the grey matter region (Fig. 2). As expected, coil E with whole head coverage outperforms others in deep brain regions in SNR. Compared to coil E, coil D with extra coverage around temporal lobes showed comparable SNR at most brain regions. Coil C with less coverage on top of the head shows comparable SNR within most brain region compared to coil D except the cortical area right behind the slot for putting head-post. Compared to Coil A, Coil B with more coverage around shoulder demonstrates higher SNR at occipital cortical area in the SNR ratio map. Viewing the acceleration performance, coil E also outperforms other coils in deep brain regions for accelerations in all directions. Except coil E, all the coils show quite similar g-factor in F-H and L-R directions, whereas significant variations in g-factor is observed in A-P acceleration direction. With extra coverage around shoulder for coil B, lower g-factor can be observed close to the neck and shoulders as compared to coil A.Discussion and Conclusion
For macaque brain imaging at 7T, five coil array configurations with different coil coverage under realistic considerations were systematically evaluated through quantifying their spatial SNR profiles and parallel imaging acceleration performance. Extending the traditional helmet coverage design for monkey head to whole-head coverage demonstrated substantial improvement in acceleration performance in deep brain region, but less pronounced enhancement can be observed in spatial SNR profiles in brain area. It suggests that the capability of parallel imaging is more sensitive to coil coverage. For occipital cortical area and deep brain, coil array with extra coverage near the temporal lobe and shoulder would be a good tradeoff between coverage constraint (head-fixation and visual stimulus) and spatial-/temporal-SNR requirement.Acknowledgements
We would like to thank Bastien Guerin for useful discussions.References
1. Chen G, Wang F, Gore J C, et al. Layer-specific BOLD activation in awake monkey V1 revealed by ultra-high spatial resolution functional magnetic resonance imaging[J]. Neuroimage, 2013, 64: 147-155.
2. Duong T Q, Kim D S, Uğurbil K, et al. Spatiotemporal dynamics of the BOLD fMRI signals: toward mapping submillimeter cortical columns using the early negative response[J]. Magnetic resonance in medicine, 2000, 44(2): 231-242.
3. Gilbert K M, Gati J S, Barker K, et al. Optimized parallel transmit and receive radiofrequency coil for ultrahigh-field MRI of monkeys[J]. Neuroimage, 2016, 125: 153-161.
4. Janssens T, Keil B, Serano P, et al. A 22‐channel receive array with Helmholtz transmit coil for anesthetized macaque MRI at 3 T[J]. NMR in Biomedicine, 2013, 26(11): 1431-1440.
5. Robin E, Laleh G, Choukri M, et al. Receive Coil Array Considerations for Simultaneous Multislice Imaging in Cardiac MRI. Proc. In proc ISMRM. 2017;25.
6. Polimeridis AG, Villena JF, Daniel L, et al. Stable FFT-JVIE solvers for fast analysis of highly inhomogeneous dielectric objects. J Comput Phys. 2014;269:280-296.
7. Villena JF, Polimeridis AG, Wald LL, et al. MARIE – a MATLAB-based open source software for the fast electromagnetic analysis of MRI systems. In proc ISMRM. 2015;23:0709.
8. Chen W, Peng B, Sun Y, et al. Subject-specific 3D Modeling of Macaque Brain via Automatic Tissue Registration Based on in vivo MR Images Acquired at 7T. In proc ISMRM. 2017;1280.
9. Pruessmann K P, Weiger M, Scheidegger M B, et al. SENSE: sensitivity encoding for fast MRI[J]. Magnetic resonance in medicine, 1999, 42(5): 952-962.
Figures