1745
A coil-noise-dominated flexible array inside a whole-head coil to improve temporal SNR in non-human primate imaging1Centre for Functional and Metabolic Mapping, The University of Western Ontario, London, ON, Canada, 2Department of Computer Science, The University of Western Ontario, London, ON, Canada, 3Department of Physiology and Pharmacology, The University of Western Ontario, London, ON, Canada
Synopsis
Typically, coil elements or arrays are dispersed on a two-dimensional surface to ensure their sensitivity profiles do not overlap, since correlated noise mitigates an SNR improvement when overlapping coils are operating in the sample-noise-dominated regime. In this study, we show that a small flexible array, operating in the coil-noise-dominated regime, can locally improve temporal SNR when placed inside a whole-head array. The two concentric arrays are inductively decoupled using preamplifier decoupling, and the contribution of coil noise to the overall noise reduces the noise correlation. Up to a two-fold increase in temporal SNR is achieved in the motor cortex.
Introduction
In non-human-primate imaging, higher channel-count arrays1,2, in close proximity to the sample, are becoming increasingly prevalent to achieve the SNR required for high-resolution anatomical and functional studies3; however, as the constituent coil elements become smaller, thermal (coil) noise accounts for a greater proportion of the overall noise with respect to noise originating from the sample4. For inductively decoupled coils operating in the coil-noise-dominated regime, their noise profiles will be essentially uncorrelated even if their sensitivity profiles overlap. In this study, we present a flexible receive array that is placed inside an enveloping whole-head coil, with both coils specifically designed for non-human-primate imaging. We demonstrate, contrary to what is typically expected for sample-noise-dominated coils, that there is a significant improvement in temporal SNR by employing two concentric arrays—one of which is coil-noise dominated—that are both sensitive to the same field-of-view.Methods
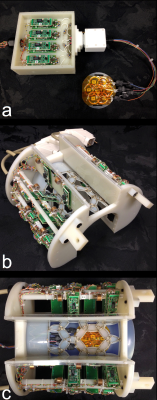
A 7-channel, flexible receive coil was constructed that could be placed inside a previously published whole-head, 24-channel receive coil for non-human primates5 (Figure 1). Each element of the flex coil was 2.5-cm wide, producing a total array width of 6.4 cm. Each element was connected to a low-input-impedance preamplifier by a coaxial cable with a half-lambda electrical length (including two lattice baluns, a phase shifter, and a high-density coaxial connector). All coils were tuned to 297 MHz.
The Q-ratios of the flex coil were measured for an isolated element and when elements were placed in the completed array with coaxial cables and lattice baluns attached. The Q-ratio of a representative element of the whole-head coil was likewise measured. The S12 between elements of the flex coil and between a representative element of the whole-head array was measured directly from the preamplifier input ports. Preamplifier decoupling—essential for reducing inductive coupling (and therefore noise correlation) between the two concentric arrays—was measured using a standard double-probe technique6.
To evaluate temporal SNR, the flex coil was placed over the motor cortex of a rhesus macaque. The whole-head coil was placed directly over top of the flex coil. The thin flex coil required only ~2.5 mm of radial space. Multi-band EPI7 time-series were acquired with both the whole-head coil and flex coil active and subsequently with just the flex coil active. The temporal SNR was calculated from the de-trended time series on a pixel-by-pixel basis. All imaging was performed on a 7T head-only scanner.
Results
The Q-ratio of an isolated flex element was 4.8. When placed in the completed array with associated cables and baluns, the Q-ratio was reduced to 1.2 – 1.4; this indicated the flex coil was operating in the coil-noise-dominated regime and coil noise accounted for 71% to 83% of the total noise. The Q-ratio of the representative whole-head coil element was 3.4, indicating that coil noise contributed only 29% of the total noise.
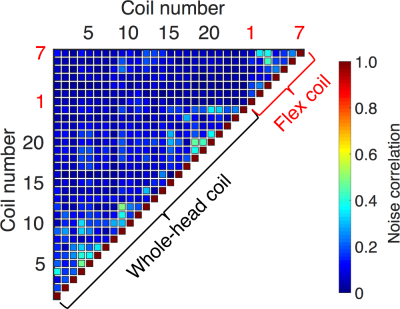
Without preamplifier decoupling, the mean S12 between elements of the flex coil was -22 dB. The worst-case isolation between an element of the flex coil and an element of the whole-head coil was approximately -8 dB; however, preamplifier decoupling provided an additional -20 dB of isolation between elements of the two arrays. This resulted in a mean noise correlation between elements of the flex coil of 18% and an overall correlation of 10% for all elements. The maximum noise correlation between any element of the whole-head coil and any element of the flex coil was 23% (Figure 2).
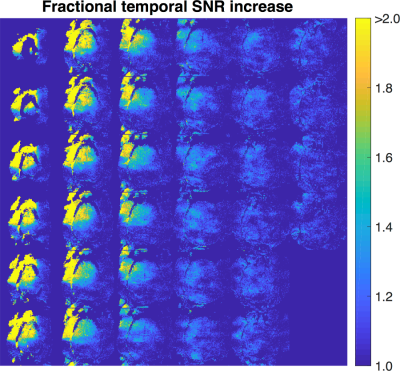
The ratio of the temporal SNR when the whole-head coil and flex coil were active versus when only the whole-head coil was active is provided in Figure 3. The temporal SNR in the motor cortex improved by up to a factor of two and improvements in temporal SNR were still realized deep within the brain.
Discussion
Preamplifier decoupling sufficiently reduced through-space inductive coupling between the flex coil and the whole-head coil. Through-sample, resistive coupling is unavoidable; however, the contribution of coil noise to the overall noise allowed the flex coil to remain largely uncorrelated with the surrounding whole-head coil. The result was a significant increase in temporal SNR, despite the sensitivity of the two arrays being entirely overlapped.
Traditionally, coil arrays are arranged on a two-dimensional surface to ensure their sensitivity profiles do not overlap, since there is diminished SNR benefit when coils are operating in the sample-noise-dominated regime. We have shown that a significant improvement in temporal SNR can be achieved by adding a local coil array that operates in the coil-noise dominated regime. This topology becomes increasingly useful when array elements become small, such as when imaging rodents and non-human primates.
Acknowledgements
No acknowledgement found.References
- Papoti D, Yen CC, Hung CC, Ciuchta J, Leopold DA, Silva AC. Design and implementation of embedded 8-channel receive-only arrays for whole-brain MRI and fMRI of conscious awake marmosets. Magn Reson Med 2017;78(1):387-398.
- Janssens T, Keil B, Farivar R, McNab JA, Polimeni JR, Gerits A, Arsenault JT, Wald LL, Vanduffel W. An implanted 8-channel array coil for high-resolution macaque MRI at 3 T. Neuroimage 2012;62(3):1529-1536.
- Logothetis NK, Merkle H, Augath M, Trinath T, Ugurbil K. Ultra high-resolution fMRI in monkeys with implanted RF coils. Neuron 2002;35(2):227-242.
- Hoult DI, Lauterbur PC. Sensitivity of the zeugmatographic experiment involving human samples. J Magn Reson 1979;34(2):425-433.
- Gilbert KM, Gati JS, Barker K, Everling S, Menon RS. Optimized parallel transmit and receive radiofrequency coil for ultrahigh-field MRI of monkeys. Neuroimage 2016;125:153-161.
- Keil B, Alagappan V, Mareyam A, McNab JA, Fujimoto K, Tountcheva V, Triantafyllou C, Dilks DD, Kanwisher N, Lin WL, Grant PE, Wald LL. Size-optimized 32-channel brain arrays for 3 T pediatric imaging. Magn Reson Med 2011;66(6):1777-1787.
- Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Ugurbil K. Multiband multislice GE-EPI at 7 Tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain FMRI. Magn Reson Med 2010;63(5):1144-1153.
Figures