1729
A 3D printed lung phantom for exploration of the limits of 19F-C3F8 ventilation imaging resolution and SNR1POLARIS, Academic Radiology, University of Sheffield, Sheffield, United Kingdom, 2GE Healthcare Inc., Aurora, OH, United States
Synopsis
Fluorinated gas imaging is a complementary method to hyperpolarized gas ventilation imaging, but suffers from lower SNR by virtue of low spin density and thermal polarisation. We present a 3D printed lung phantom based on a gold standard lung ventilation scan acquired from 3He MRI used to explore the limits of fluorinated gas MR in terms of spatial resolution and SNR. Images acquired with unrealistically long imaging times for in-vivo exams were compared to lower resolution images. The results demonstrate that resolutions obtainable with in-vivo fluorinated gas imaging miss potentially important spatial variation information.
Background
Fluorinated gas imaging is a promising method for assessment of lung ventilation in obstructive lung diseases1,2. However, the substantially weaker MR signal when compared to hyperpolarized gas MRI3 requires the acquisition of much lower resolution images for sufficient signal-to-noise ratio. Without comparing 19F lung ventilation images of similar resolution to those obtained with hyperpolarized gas it is not possible to determine if fluorinated gas imaging can provide a clinically useful and potentially cheaper alternative. Previously, 3D printed anatomical phantoms of the lung4 heart5, breast, brain6 have been developed for rapid evaluation of MR techniques and methods that could not be practically or safely studied through in-vivo study. In these models it is desirable to reproduce the structural and MR parameters as closely as possible, to enable valid comparisons with expected in-vivo results.Purpose
To design and image 3D printed lungs for verifying methods of 19F ventilation imaging. With a lung model containing suitable spatial structure we aimed to compare the lower-resolution imaging obtainable in-vivo with 19F ventilation imaging to higher-resolution imaging typically obtained with hyperpolarized gas imaging, but not practically achieved in-vivo with 19F MRI due to constraints of in vivo scanning time.Methods
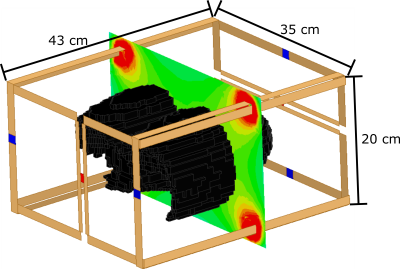
Using a a flexible transmit/receive vest coil (CMRS, Brookfield, Wisconsin, USA) on a 1.5T GE Signa HDx scanner, a 3D 3He ventilation image with the desired imaging resolution was obtained as a gold-standard data set (150 ml 3He, 3D steady-state free precession, BW = ±43 KHz, FA = 15°, TE = 0.8 ms, TR = 2.0 ms, 100×82×24 matrix, 4x4x10 mm3 resolution). A 3D volumetric surface was derived by median filtering the image, then excluding voxels in the model with neighbouring voxels with intensity greater than the threshold of noise. The shell was printed with a Dimension SST 1200 esTM 3D printer using a substrate polymer (ABS) with MRI compatible EM properties. Then, 4 mm tubing was inserted in the lung model airspaces to represent small vessels/arteries and defects that may be observed in diseased lungs. A homemade 19F coil with two sets of parallel coils driven in quadrature was designed and built for homogeneous excitation and reception (model shown in Figure 1 with the 3D printed lung model).
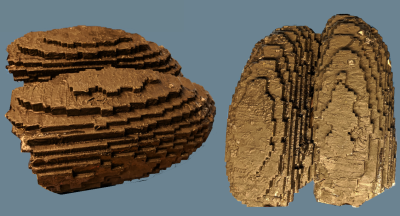
The lung phantom (shown in Figure 2) was filled with 80% C3F8 and 20% O2 then imaged with the following parameters: 3D steady-state free precession, TE = 2.5 ms, TR = 5.3 ms, BW = ±11.9 KHz, FA = 90°, 100x82x24, 4x4x10 mm3 resolution and 80 averages for a resulting 13:54 minutes of imaging. A 19F image was also acquired with half the spatial resolution in all three Cartesian dimensions, half the acquisition bandwidth and half the averages resulting in a 1:44 minute acquisition. The k-space of low-resolution images were zero-filled to the equivalent size as high resolution images. The three images were compared in terms of visual appearance and SNR. Additionally, the images were also compared with the coefficient of variation (CV) as a metric of the spatial variation within the phantom, calculated as the standard deviation within a 3x3 in-plane region of interest around each voxel divided by the mean7. Maps were also derived from the images where the CV was between 0.15<0.35 and >0.35 to highlight the accuracy in reproducing medium and high variation regions, respectively.
Results
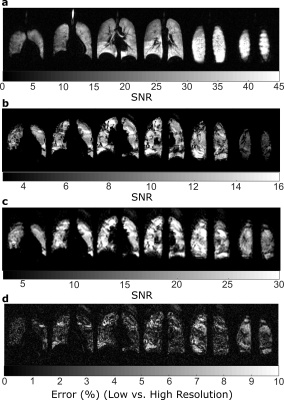
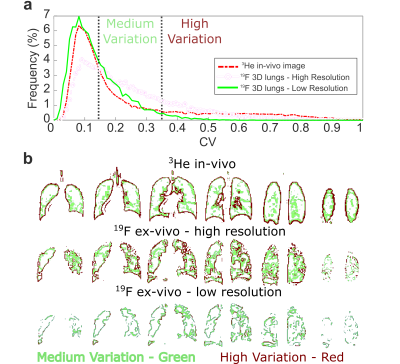
Images of representative slices from 3He in-vivo imaging and 19F imaging of the 3D printed phantom are shown in Figure 3. Vessels are not well reproduced, but the variation from tubing closely matches what could be expected from volume defects; potentially from masses or from unfilled airways. Percent error shown in Figure 3c demonstrate that the low resolution images miss many of the ‘defect’ regions. In Figure 4a the CV histograms show lowered median CV and a narrowed distribution. Also, in Figure 4b masks of the different levels of CV show that the accuracy of extracting edges is greatly reduced.Discussion
The 3D printed lung phantom allows investigation of 19F gas imaging resolutions greater than achievable in-vivo. Despite some visual ‘blockiness’ of the printed lungs resulting from the spatial resolution of the 3He gold standard data set, the resolution of imaging is equivalent so that in MR imaging it is not apparent.Acknowledgements
Doctoral program funding for Adam Maunder was partially provided by support from GE Healthcare Inc. and scholarships from the Natural Sciences and Engineering Research Council of Canada (NSERC) and University of Sheffield. This work was funded by the National Institute for health research (NIHR), Medical Research Council (MRC) and University of Sheffield Hyperpolarised Imaging Group - POLARIS. The views expressed in this abstract are those of the author and not necessarily those of NHS, NIHR, MRC or the Department of Health.References
1. A. F. Halaweish, R. E. Moon, W. M. Foster, B. J. Soher, H. P. McAdams, J. R. MacFall, et al., "PErfluoropropane gas as a magnetic resonance lung imaging contrast agent in humans," Chest, vol. 144, pp. 1300-1310, 2013.
2. M. J. Couch, I. K. Ball, T. Li, M. S. Fox, A. V. Ouriadov, B. Biman, et al., "Inert fluorinated gas MRI: a new pulmonary imaging modality," NMR in Biomedicine, vol. 27, pp. 1525-1534, 2014.
3. M. Couch, B. Blasiak, B. Tomanek, A. Ouriadov, M. Fox, K. Dowhos, et al., "Hyperpolarized and Inert Gas MRI: The Future," Molecular Imaging and Biology, vol. 17, pp. 149-162, 2015/04/01 2015.
4. F. L. Giesel, A. Mehndiratta, H. von Tengg-Kobligk, A. Schaeffer, K. Teh, E. A. Hoffman, et al., "Rapid Prototyping Raw Models on the Basis of High Resolution Computed Tomography Lung Data for Respiratory Flow Dynamics," Academic Radiology, vol. 16, pp. 495-498, 2009/04/01/ 2009.
5. S. A. Chikop, A. S. Konar, N. S. L. Reddy, N. Vajuvalli, D. S. Keelara, A. Kumnoor, et al., "A Cost-effective 3D Printed Cardiac MR Phantom," Proc. Int. Soc. Magn. Reson. Med., vol. 25, p. 2765, 2017.
6. K. Gopalan, J. I. Tamir, A. C. Arias, and M. Lustig, "Toward 3D Printed, Anatomy-Mimicking, Quantitative MRI Phantoms," Proc. Intl. Soc. Mag. Reson. Med., vol. 25, p. 3733, 2017.
7. P. J. C. Hughes, F. C. Horn, G. J. Collier, A. Biancardi, H. Marshall, and J. M. Wild, "Spatial fuzzy c-means thresholding for semiautomated calculation of percentage lung ventilated volume from hyperpolarized gas and 1H MRI," J Magn Reson Imaging, Jul 06 2017.
Figures