1705
Large FOV 16-channel receive array with a volume transmit coil for human forearm/wrist/hand imaging at 7 T1CIBM-AIT, Ecole Polytechnique Federale de Lausanne, Lausanne, Switzerland, 2LIFMET, Ecole Polytechnique Federale de Lausanne, Lausanne, Switzerland, 3Neuroradiology division, Geneva University Hospitals, Geneva, Switzerland
Synopsis
A large-field-of-view 16-channel circular loop receive array with a volume transmit coil for the human forearm, wrist and hand imaging at 7 Tesla was constructed. While the volume transmit coil yields homogeneous transmit field distribution along the 350-mm in length , the 16-channel receiver array enables two times faster imaging with a similar MR image quality. In conclusion, the use of this large field-of-view RF coil configuration for a total MR protocol of 15 minutes is feasible, and it enables visualization of different anatomical structures on the human forearm and hand at 7 Tesla.
Introduction
High-resolution imaging of small size anatomical structures like the human wrist, hand or arm is beneficial at 7 Tesla because the increased SNR could be directly converted into higher spatial resolution1-2. Moreover, the use of multi-channel receivers provides the opportunity to use parallel imaging techniques, which is resulted in a substantial decrease in acquisition time3-6. A combination of multi-channel receivers with 350 mm-long volume coil to image the human forearm, wrist, hand and fingers could be quite advantageous to scan all these extremities once with high acceleration factor. The goals of this study were: (i) to determine the feasibility of performing high-resolution MRI of the wrist and hand at 7T using a sixteen-receive array and 350mm-long volume-transmit coil and parallel imaging, (ii) to evaluate how the implementation of higher acceleration factors affects image quality, as measured as SNR.Methods
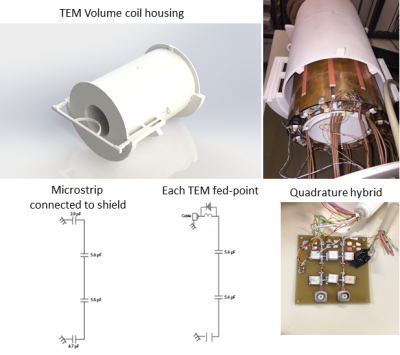
A 12-microstrips shielded (350-mm long, 200-mm diameter) TEM volume transmit coil with an inner diameter of 150 mm and a length of 350 mm was constructed (Fig.1). Each microstrip (350-mm long, 25mm-wide) was interspersed with two capacitors of 5.6 pF each, to reduce to a maximum of 116 mm conductor length, each. 3.9 pF and 4.7 pF capacitors connected each microstrip to the shield. TEM volume coil matched to 50Ω at 297 MHz was driven using quadrature hybrid. Four PIN diodes were added on the ports and two microstrips centered on two loop coil array rows. Safety parameters were simulated as in the experimental setup on the posable Duke model7 (Virtual Family) with FDTD simulations (Sim4Life 3.4.0.2195, ZMT, Switzerland). The average SAR observed was 1.03 W/kg per 10g of tissue.
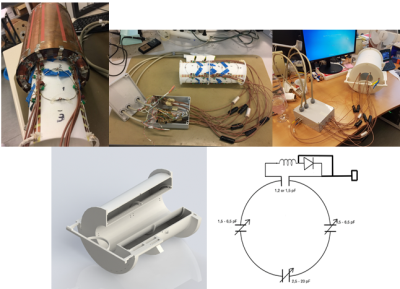
16-element circular loop receivers of diameter of 80 mm were distributed over a 3D-printed cylinder of diameter 135 mm and length 350 mm along the volume coil, in four rows of four elements each (Fig.2). Neighboring elements were overlapped to minimize coupling between them. The elements were actively decoupled during transmission and were passively decoupled along with fuses incorporated as a safety measure (Fig.2). The preamplifiers were placed in a separate housing. As the electrical length between the elements and the preamplifiers was high (λ7T,air/3), multiple cable traps were placed in the RF lines to reduce additional coupling between them. Scattering parameters were assessed with a network analyzer (Agilent E5071C, Santa Clara, CA) in the loaded condition with the bottle phantom. A cylindrical bottle water phantom (80-mm diameter, 300 mm in length) was scanned on a 7T head-only MRI scanner (Siemens Healthineers, Erlangen) to determine B1+8, SNR and g-factor maps. G-factor maps were generated by acquiring 2D gradient-echo images (TR/TE=300/7.8ms, BW= 120 Hz/Px, flip angle=30 ̊, FOV=250x250mm2, matrix=256x256) in sagittal orientations. An additional full k-space scan was obtained with no RF excitation for determination of noise statistics. Accelerated data sets were generated by omitting phase-encoding lines using GRAPPA9 with effective acceleration factors of R= 1.8, 2.4 and 2.8 (using 32 reference lines). Finally g-factor maps10 were calculated pixelwise by g-factor=SNRfull/(SNRaccel*√R). Accelerated 3D-GRE images were acquired (TR/TE=60/3ms, BW=400 Hz/Px, flip angle=30 ̊, FOV=230x230mm2, matrix=128x128) with Reff ranging from 1.7 to 3.8.
The subjects were scanned in the prone position with the wrist extended in front of them (“superman” position). Proton density 3D turbo spin echo (TSE) images were acquired at 0.5x0.5x2.0 mm3, with TR/TE/TA = 3000ms/40ms/4min. 3D T1-weigted TSE were acquired at a resolution of 0.4x0.3x1.0 mm3, with TR/TE/TA=903ms/10ms/5min.
Results
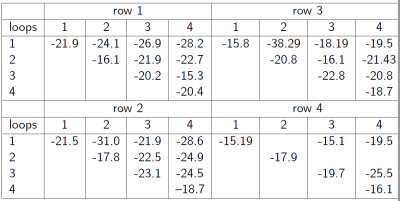
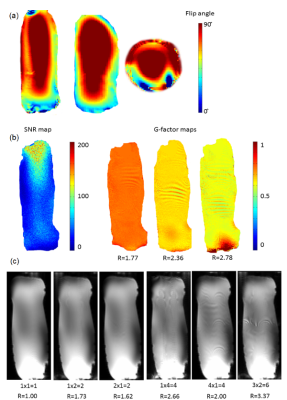
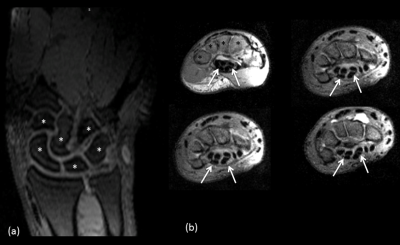
The scattering parameters of the 16-channel receiver loops are shown in Fig.3. The average reflection coefficient was above -16dB and the coupling between loops was above -15dB. B1+ maps of the TEM volume coil shows rather homogeneous flip angle distribution with a maximum at the center of the TEM coil along the 300-mm long bottle phantom (Fig.4a). Mean g-factors were 0.75, 0.64 and 0.59 for Reff=1.8, 2.4 and 2.9, respectively(Fig.4b). While the 3D GRE images show similar SNR with grappa factor 2 and no grappa factor, the images depict signal distortion with the grappa factor 4 (Fig.4c). High-resolution anatomical images show clear delineation of cartilage on the hand and median nerve in the wrist (Fig.5).Discussions and conclusions
The results demonstrate the feasibility of the 16-channel Rx array with a TEM volume Tx for a large FOV human arm and hand imaging at 7T. Our developed RF array was capable of running TSE sequences which is power demanding and sensitive for B1+ field inhomogeneity with high acceleration factors. Our total MR protocol time of 15 minutes leads to decreased motion artifact, greater flexibility in MRI sequence design, and greater patient comfort due to decreased acquisition time.Acknowledgements
This study was supported by Centre d’Imagerie Biomedicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL, the Leenaards and Jeantet Foundations and startup subsidy department of Radiology. Authors thank to Matthieu Chabanel, Angel Chauffray and Jose Giacomini for their work in coil construction.References
1. Friedrich K.M., Chang G., Vieira R.L.R., Wang L., Wiggins G.C., Schweitzer M.A., Regatte R.R. In vivo 7.0-Tesla magnetic resonance imaging of the wrist and hand: technical aspects and applications. Semin Musculoskelet Radiol. (2009); 13(1):74-74.
2. Raval S.B., Zhao T., Krishnamurthy N., Santini T., Britton C., Gorantla V.S., Ibrahim S.T. Ultra-high field RF coil development for evaluating upper extremity imaging applications, NMR Biomed. (2016); 29: 1768-1779.
3. Kraff O., Bitz A.K., Dammann P., Ladd S.C., Ladd M.E., Quick H.H. An eight-channel transmit/receive multipurpose coil for musculoskeletal MR imaging at 7 T, Med. Phys. (2010); 37 (12), 6368-6376.
4. Raval S.H., Britton C.A., Zhao T., Krishnamurthy N., Santini T., Gorantla V.S., Ibrahim T.S. Ultra-high field upper extremity peripheral nerve and non-contrast enhanced vascular imaging. Plos one (2017).
5. Raghuraman S., Mueller M.F., Zbyn S., Baer P., Breuer F.A., Friedrich K.M., Trattnig S., Lanz T., Jakop P.M. 12-channel receive array with a volume transmit coil for hand/wrist imaging at 7 T. JMRI (2013); 38: 238-244.
6. Chang G., Friedrich K.M., Wang L., Vieira R.L.R., Schweitzer M.E., Recht M.P., Wiggins G.C., Regatte R.R. MRI of the wrist at 7 Tesla using an eight-channel array coil combined with parallel imaging: preliminary results JMRI (2010); 31:740-746.
7. Gosselin M.C., Neufeld E., Moser H. et al. Development of a new generation of high-resolution anatomical models for medical device evaluation: the Virtual Population 3.0. Phys. Med. Biol. (2014); 59(18): 5287.
8. Eggenschwiler F, Kober T., Magill A.W. et al. Sa2RAGE: a new sequence for fast B1+ -mapping. Magn. Reson Med. 2012; 67(6):1609-19.
9. Griswold M.A., Jakop P.M., Heidemann R.M., Nittka M., Jellus V., Wang J., Kiefer B., Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med (2002);47(6):1202-10.
10. Pruessmann K.P., Weiger M., Scheidegger M.B., Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. (1999);42(5):952-62.
Figures