1678
Monte Carlo simulations of diffusion in myelin spirals: Impact on diffusional water exchangeLorenza Brusini1, Gloria Menegaz1, and Markus Nilsson2
1University of Verona, Verona, Italy, 2Lund University, Lund, Sweden
Synopsis
How does the myelin structure impact water diffusion? The answer is still not clarified but is important for interpreting diffusion MRI in conditions with altered myelin structure such as neurological disorders or developing brain. Myelin is sometimes modelled as permeable to explain exchange between compartments. This work investigates the impact of the spiralling nature of myelin on water exchange, until now only indirectly explored in one case. Findings emphasized that small axons and low number of myelin wraps lead to exchange times shorter than a second, which can be assessed at clinical scanners.
INTRODUCTION
Myelin is a membranous structure that wraps axons with multiple layers 1,2. Due to its short transverse relaxation time (T2) 3, its contribution is often neglected in models of the diffusion MRI signal. However, the exchange of water across the myelin may be quantified with diffusion MRI. This work aims at investigating the impact of the multi-wrapping nature of myelin on the exchange of water and on the dMRI signal.METHODS
Water exchange times ($$$\tau$$$) were assessed for different configurations of spiralling myelin using Monte Carlo simulations. The simulation substrate was composed of three compartments: axon, myelin, and extracellular tissue, as in Fig. 1. Particles were allowed to diffuse in the xy-plane in the axon/extra-axon space, while in myelin they were forced to diffuse in a one-dimensional spiralling compartment (axon diameter $$$\sim 1$$$ $$$\mu$$$m 4, myelin extracellular space width $$$\sim 30$$$ nm 2). The length of the spiral was calculated from the number of myelin wraps and the axon diameter. Particles were allowed to enter or exit the myelin spiral at two specific points on in the intra- and extra-axonal spaces. Impact of simulation geometry on exchange times was investigated in two cases, that is by varying the number of wraps ($$$n_{wraps}=1,2,4,8,16,32$$$ for $$$d_{inner}=1,2$$$ $$$\mu$$$m) and the inner axon diameters ($$$d_{inner}=1,2,4$$$ $$$\mu$$$m for $$$n_{wraps}=1,4$$$), respectively. Exchange times were assessed by two methods. First, the number of intra-axonal particles that stayed within the axons ($$$n$$$) was recorded as a function of time for 200 ms. The exchange time was then computed via polynomial fitting in a least-squares sense from the equation: $$$n\left(t\right)=n_{0}\exp\left(\frac{-t}{\tau}\right)$$$. Second, the MR signal was simulated for $$$\delta=15$$$ ms, $$$\Delta=25,55,255$$$ ms with b-values in the range 0-2500 s/mm$$$^2$$$ and Gaussian noise (SNR = 40) was added in 500 independent realisations, to quantify the variability in the estimated parameters. Exchange times, intra-axonal volume fractions and extracellular diffusivities were estimated by fitting the Kӓrger model to the data 5.REULTS
Figure 2A and 2B show exchange times versus number of wraps and versus axon diameter, respectively, showing that exchange time increases with both the axon diameter and the number of myelin wraps. Figure 3 shows the parameters estimated via Kӓrger model fitting. Intra-cellular volume fraction increased with the number of myelin turns, while the extra-cellular apparent diffusion coefficient was insensitive to it. Exchange time estimated via the Karger model was in agreement with the trend observed in Figure 2, featuring an increase with the number of wraps. The fitting error also increased with the number of wraps. Importantly, exchange times recovered for small axons with less than 8 myelin turns were close to the expected values.DISCUSSION
In a simple simulation substrate with myelin implemented as a long spiral, the exchange times increased with both the axon diameter and the number of wraps. In particular, exchange times of more than one second were observed for axons larger than 1 micron or with more than 8 wraps, in agreement with expectations from Nilsson et al 6. Since the large majority of axons have diameter below 1 micron 7, our results suggest that clinically-observable exchange times on the sub-second scale could be observed in-vivo when the numbers of wraps are lower than approximately 10. According to Edgar et al 1, the number of myelin wraps in human brain is normally around 10, which gives exchange rates outside of the observable range. However, diminished number of myelin wraps are found in the developing and degenerating brain 8,9. Studying exchange may thus open a new route for probing the myelination and demyelination processes.CONCLUSION
This study highlights the dependence of the exchange time on the axon inner diameter and the width of the myelin sheet. Small axons and low number of myelin turns could yield sub-second exchange times. Such conditions could be found in the infant brain and in demyelinating diseases. Resulting exchange times could be assessed using clinical acquisitions, potentially revealing useful information for development- and disease-related processes.Acknowledgements
No acknowledgement found.References
- Edgar J M, Griffiths I R. Chapter 7 - white matter structure: A microscopists view. Diffusion MRI (Second Edition), second edition ed., Johansen-Berg H, Behrens T E. Eds. San Diego: Academic Press. 2014; pp. 127 – 153.
- Paus T. Growth of white matter in the adolescent brain: myelin or axon? Brain and cognition. 2010;72(1):26–35.
- Whittall K P, Mackay A L, Graeb D A, Nugent R A, Li D K, Paty D W. In vivo measurement of t2 distributions and water contents in normal human brain. Magnetic resonance in medicine. 1997;37(1):34–43.
- Innocenti G M, Caminiti R, Hof P R. Fiber composition in the planum temporale sector of the corpus callosum in chimpanzee and human. Brain structure and function. 2010;215(2):123–128.
- Kӓrger J. Principles and applications of self-diffusion measurements by nuclear magnetic resonance. Adv Magn Reson. 1988;12:1–89.
- Nilsson M, Lätt J, van Westen D, Brockstedt S, Lasič S, Ståhlberg F, Topgaard D. Noninvasive mapping of water diffusional exchange in the human brain using filter-exchange imaging. Magnetic resonance in medicine. 2013;69(6):1572–1580.
- Liewald D, Miller R, Logothetis N, Wagner H-J, Schüz A. Distribution of axon diameters in cortical white matter: an electronmicroscopic study on three human brains and a macaque. Biological cybernetics. 2014;108(5):541–557.
- Chang K-J, Redmond S A, Chan J R. Remodeling myelination: implications for mechanisms of neural plasticity. Nature neuroscience. 2016;19(2):190.
- Albert M, Antel J, Brück W, Stadelmann C. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathology. 2007;17(2):129–138.
Figures
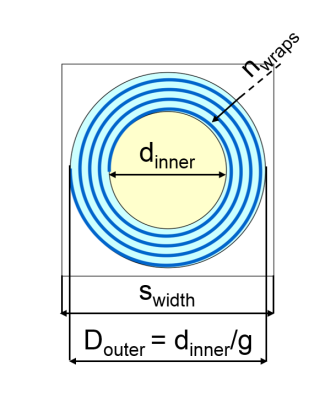
Substrate
composed of three compartments: axon (yellow), myelin (blue), extra-axon (white).
The variables of the substrate are the width of the square unit (swidth),
the axon diameter (dinner), the ratio between the inner and outer diameters
of the structure comprehensive of axon and myelin (g) and the number of myelin
turns (nwraps)
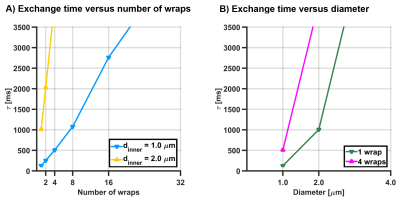
Exchange time observed from the average
time elapsed until particles to escape from the axon into the extra-axonal
space, versus (A) the number of wraps for axon diameters of 1 and 2 micron, and (B) the axon diameter for 1
and 4 myelin wraps.
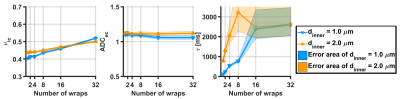
Estimated parameters
from the Kӓrger, for axon diameters of 1
and 2 micron:(A) intracelullar volume
fraction, (B) extracellular apparent diffusion coefficient and (C) exchange time.
Areas indicates the 5th and 95th percentiles computes from 500 noisy instances
of the simulated signal (SNR = 40).