1676
Diffusion Weighted Imaging with uniform fat suppression using a Modified Dixon based Single Shot Turbo Spin Echo1Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 2Philips Research, Hamburg, Germany, 3Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 4Kidney Cancer Program, Simmons Comprehensive Cancer Center, UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
Diffusion weighted imaging using single-shot turbo spin echo (DW-SShTSE) with Dixon showed uniform fat suppression without geometric distortions, compared to DW-EPI and DW-SShTSE with spectrally selective fat suppression (SPIR). However, the phase insensitive preparation used in DW-SShTSE reduces the SNR by half, impeding the robustness of Dixon reconstruction. In this work, we developed a hybrid DW-SShTSE, where the b=0 s/mm2 image was acquired without the phase insensitive preparation for improved SNR. This combined with modified acquisition order improved the robustness of fat/water separation and generated diffusion-weighted images of the cervical spine with improved spatial resolution.
Introduction
In areas with large B0 inhomogeneities, such as spinal cord, single-shot turbo spin echo (SShTSE) based diffusion weighted imaging (DW-SShTSE) is increasingly used due to its robustness to geometric distortions1. DW-SShTSE is commonly acquired with spectrally selective inversion recovery (SPIR) to suppress fat for improved conspicuity of lesions. While SPIR provides higher SNR compared to STIR, it suffers from incomplete fat suppression in areas with large B0 inhomogeneities. Previously, we developed a DWI-SShTSE using a multi-echo Dixon combined with shared-B0-map reconstruction for uniform fat suppression without the need for additional image navigators2. However, the reduced SNR in high-resolution DWI and/or with long diffusion preparation impedes the robustness of fat/water separation, even with the advanced reconstruction methods3,4. Thus, the purpose of this work was to develop strategies for improved fat/water separation to generate high-quality DWI images with uniform fat suppression and improved spatial resolution.Theory
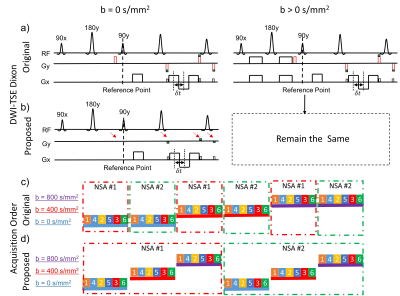
The previously proposed DW-SShTSE-Dixon (fig. 1a) utilized the phase insensitive diffusion weighting preparation5, followed by shared field-map between the b=0 s/mm2 image and the higher b-value images for robust fat/water separation across all b-values. However, the additional dephasing gradient that was employed to eliminate the non-CPMG component with the phase insensitive preparation decreased the original signal by half, reducing the overall SNR. Since there is no diffusion gradient applied for b=0 s/mm2 image, the dephasing gradient can be safely removed to regain the original signal for improved SNR and robust fat/water separation. We refer this as the hybrid DWI-SShTSE Dixon sequence, in which the images at b = 0 s/mm2 and non-zero b-values are acquired without and with the dephasing gradient respectively (fig.1b). In the conventional DWI, the apparent diffusion coefficient (ADC) is calculated as: $$ \mathrm{ADC} = \frac{{\mathrm{ln}(\frac{S_0}{S_1})}}{b_1-b_0} \quad(1)$$ where $$$S_0$$$ and $$$S_1$$$ are the signal intensities at $$$b_0$$$ and $$$b_1$$$. However, without the dephasing gradient, the signal obtained with the hybrid DWI-SShTSE-Dixon at b=0 s/mm2 will be doubled. Thus, the modified ADC can be calculated as:$$ \mathrm{ADC} = \frac{{\mathrm{ln}(\frac{S_0}{2S_1})}}{b_1-b_0} \quad(2)$$To minimize the effect of B0 drifting6 on the shared-B0-map reconstruction, the image acquisition was interleaved by signal average (NSA, fig.1d) instead of b-values (fig.1c) to shorten the gap between different b-values images.Methods
The sequence (fig.1) was implemented on a 3T Ingenia scanner (Philips Healthcare, Best, The Netherlands). Phantoms with sucrose concentrations of 0.3, 0.6, 1.2 M and peanut oil were used to validate the ADC measurements using the hybrid DWI-SShTSE Dixon. The proposed method was evaluated and compared against DW-EPI, DW-SShTSE with SPIR and Dixon in the spinal cord of 3 healthy volunteers with IRB approval and written informed consent. The typical imaging parameters of the proposed sequence included: sagittal orientation; FOV = 220×220mm2; Slice Thickness = 4mm; and directions=3. The acquisition voxel size was 2×2mm2 and 1.3×1.3mm2 for the low and high resolution DWI respectively. For 13 slices, 3 b-values (e.g. 0, 400 and 800 s/mm2) and 6 NSA, the total scan time was approximately 5 and 8 min for the low and high resolution DWI respectively.Results
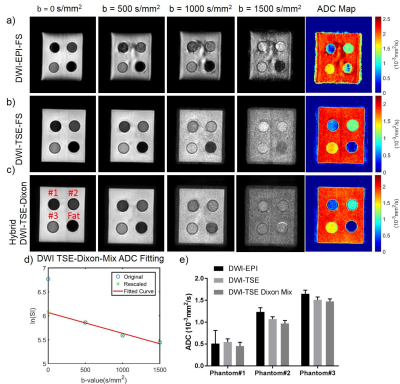
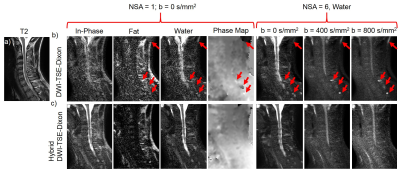
Figure 2 demonstrates the correct ADC measurement achieved by the hybrid DW-SShTSE-Dixon using Eq.2 with the scaling factor of 2 at b=0 s/mm2 (fig.2d). Compared to DW-EPI (fig.2a) and DW-SShTSE with SPIR fat suppression (fig.2b), the proposed hybrid DW-SShTSE-Dixon generated uniform fat suppression and similar ADC map (fig.2c) showing good agreement in ADC values (fig.2e). Figure 3 shows improved fat/water separation with the hybrid DW-SShTSE-Dixon compared to the original DW-SShTSE-Dixon. Fat/water swaps, mimicking lesions at higher b-value images were observed in the original DW-SShTSE-Dixon images (fig.3a, red arrows) due to the inaccurate phase estimation at b=0s/mm2. Uniform fat/water separation was achieved with the proposed hybrid DW-SShTSE-Dixon at b=0s/mm2, which further improved the fat/water separation at higher b-values with the shared-field-map Dixon reconstruction.
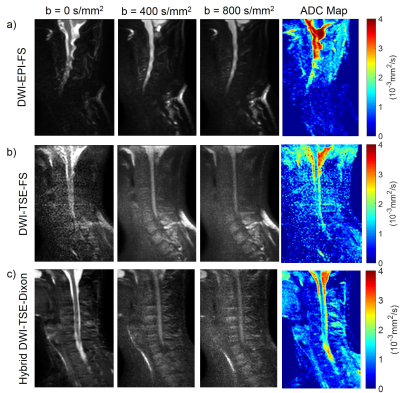
The single-shot DW-EPI suffers from severe geometric distortions (fig.4a), resulting in inaccurate ADC map. DW-SShTSE with SPIR achieved negligible geometric distortion, but suffers from nonuniform fat suppression and low SNR at b=0 s/mm2 due to the dephasing gradient (fig.4b). Compared to these techniques, the proposed hybrid DW-SShTSE-Dixon generated high SNR images at b=0s/mm2, and achieved robust fat/water separation and accurate ADC maps (fig.4c).
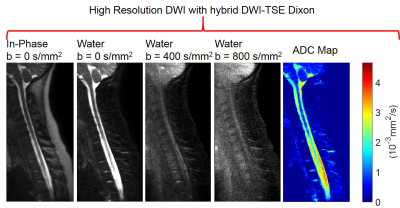
With the improved SNR at b=0s/mm2, DW-SShTSE-Dixon enables robust fat/water separation and allows high-resolution diffusion weighted images of the cervical spine (fig.5).
Discussion
We have demonstrated a hybrid DW-SShTSE-Dixon with shared-field-map to achieve robust diffusion-weighted images with uniform fat/water separation and accurate ADC measurements. This approach allows higher SNR images with negligible geometric distortions that can be used in challenging areas with increased B0 inhomogeneities such as spinal cord imaging and body applications.Acknowledgements
No acknowledgement found.References
1. Stroman PW, Wheeler-Kingshott C, Bacon M, et al. The current state-of-the-art of spinal cord imaging: methods. Neuroimage. 2014;84:1070-81.
2. Wang X, Eggers H, Pinho MC, Pedrosa I, Lenkinski RE, Madhuranthakam AJ. Diffusion Weighted Imaging using a Dixon based Single Shot Turbo Spin Echo. In Proceedings of the 25th Annual Meeting of ISMRM. Honolulu, Hawaii, USA, 2017. 0172.
3. Eggers H, Brendel B, Duijndam A, Herigault G. Dual‐echo Dixon imaging with flexible choice of echo times. Magnetic resonance in medicine. 2011;65(1):96-107.
4. Ma J, Son JB, Hazle JD. An improved region growing algorithm for phase correction in MRI. Magnetic resonance in medicine. 2016;76(2):519-29.
5. Alsop DC. Phase insensitive preparation of single‐shot RARE: Application to diffusion imaging in humans. Magnetic resonance in medicine. 1997;38(4):527-33.
6. Vos SB, Tax CM, Luijten PR, Ourselin S, Leemans A, Froeling M. The importance of correcting for signal drift in diffusion MRI. Magnetic resonance in medicine. 2017;77(1):285-99.
Figures