1672
Novel Multi-band accelerated, Reference-less, Multifaceted Icosahedral and Multishell Diffusion MRI Protocol for human whole brain clinical applications1Diagnostic and Interventional Radiology, UThealth, Houston, TX, United States, 2Neurology, UThealth, Houston, TX, United States
Synopsis
We describe a comprehensive multishell and multifaceted icosahedral diffusion MRI protocol that enables whole brain coverage in less than 10 minutes using multiband (MB) technology at 3 T. We show the protocol utility in providing estimates of blood fraction, extent of CSF-contamination, diffusion tensor and kurtosis derived measures including fractional, axonal water fraction and extracellular tortuosity. The diffusion gradient encoding is based the Icosa6 and Icosa15 sets forming the Icosa21 for additional quality assurance. In this report we describe the protocol, show feasibility and utility for mapping a host of useful quantitative measures in the same session without repeated scans.
Introduction
Diffusion-weighted MRI has advanced knowledge of the organization and functioning of the central nervous system in both health and disease. Three clinically-used dMRI quantitative models including intravoxel incoherent motion, IVIM1, diffusion tensor, DT2, and diffusion kurtosis, DK3 have been explored at low, medium and high b-values, respectively. IVIM measurements (b<300 s.mm-2) provide information about micro capillary blood volume fraction, DTI using b<1200 s.mm-2 provides scalar and orientation maps while DKI provides clues about restricted diffusion utilizing a minimum of two shells b>=1000 and b<3000 s.mm-2. Due to time considerations for clinically feasible scans, these models have not been consolidated. DTI uses a single shell (b~1000 s.mm-2), DKI uses two shells, and both require a reference or b=0 map to decouple the water content and relaxation effects from attenuation by random translational diffusion. The b=0 map contains contributions from the blood fraction, cerebrospinal fluid (CSF) and free water in the voxel may also contaminate the signal which affect the accuracy of the sought maps4. Utilizing a reference-less dMRI with b>300 s.mm-2 minimizes blood and reduces CSF contamination as an alternative to fluid-attenuation by inversion recovery5.
Methods
Whole brain data were collected on two healthy adults using a Philips Ingenia 3T system and a 32-Channel RF coil with multiband technology. dMRI protocol utilized a single-shot echo-planar sequence with a SENSE factor=2 and MB=2 for acquiring data with b=0, three shells using diffusion gradient pulses along the orthogonal X/Y/Z orientations and Icosa6 at b=50, 100, 200 s mm-2, respectively. Three additional shells with Icosa21 at b=400, 1200, 2200 s mm-2, isotropic voxel size 2mm, TR/TE=6000/90 ms; see Figs. 1, 2). Pseudo-continuous arterial spin labeling (pCASL), multi spin-echo T2w, and high spatial resolution multi gradient-echo susceptibility-weighted data were also collected to estimate cerebral blood flow, iron-rich gray matter, and vessels while also collecting phase maps. Data Processing: The averaged b=0 volume was used for motion and distortion corrections. DWI data were processed in multi-passes using the encoding b-matrix formalism for both DTI 2, 8-10 and DKI as detailed elsewhere 3,11,12 with b=0 and b>=400 to obtain asymptotic estimates for the single tensor and blood fractions1 and subsequently fit using linear and non-linear mixed Gaussian multi-exponential models. DKI was implemented using b=400, 1200, and 2200 shells with and without utilizing the b=0 map. The data were also run through windows-based freely available dMRI packages that use DTI (DTIstudio) or beyond (NODDI, DK-Estimator, Trackvis, ExploreDTI, DSIStudio; see tabulation and web links13).
Results
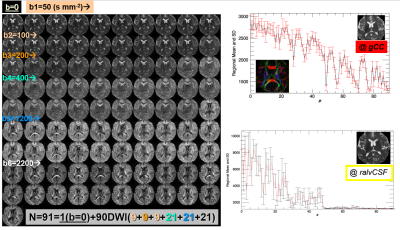
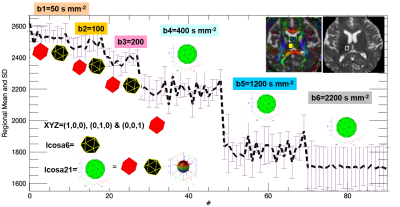
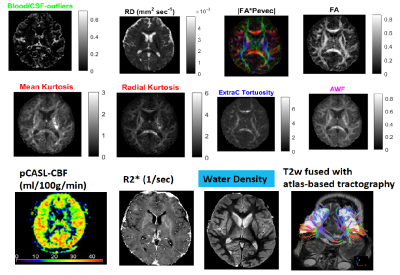
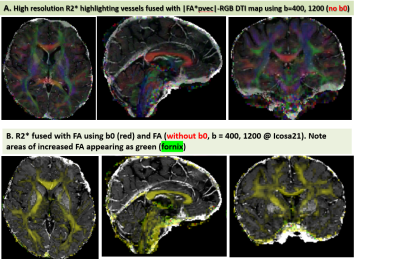
Figure 1 shows the data quality represented by one axial section and the signal profiles (means and standard deviations vs. order) in white matter (genu of corpus callosum) and CSF at the right anterior horn of lateral ventricle. The protocol design is illustrated in Figure 2 where the signal profile is shown in the right thalamic gray matter. Figure 3 shows the IVIM fraction, DT radial diffusivity, principal eigenvector, fractional anisotropy, mean & radial kurtosis, extracellular tortuosity, and axonal water fraction (AWF). The CBF map, R2*, water content, and a 3D volume render of the motor cortices and projection motor pathway traced and visualized using the dMRI data (http://dsi-studio.labsolver.org/). Figure 4.a fuses R2* map highlighting veins with the DTI-derived |FA*pvec|. Note the extent of vessel contamination on the posterior corpus callosum. Figure 4.b fuses R2* with FA derived w/o b0 keeping the b=400,1200@Icosa21. An increase in FA is shown in green and is best seen in regions surrounded by CSF (i.e. fornix). This interesting effect has been described in past DTI literature utilizing FLAIR or CSF/free water modeling.14,15
Discussion
A minimum of 6 DWI plus one b0 are needed to map the single diffusion tensor2, whereas 15 independent measurements are needed to determine the kurtosis tensor3, hence a minimum of 22 measurements are required for combined single DT and DK estimation3. These minimum requirements are not sufficient to obtain the free water or blood fraction without adhoc assumptions or constraints6,10-12. Combinations of XYZ, Icosa6 and Icos15 of b>=400 values can be subsampled to obtain DTI and DKI-derived measures, hence providing estimates at different SNR levels from the same data. The use of DWI with b>=400 s.mm-2 minimized contamination from blood, improved accuracy and reproducibility of the DTI and DKI maps.Conclusion
Extracting the blood fraction in the voxel offers a non-invasive dMRI method to monitor conditions or interventions that alter the vasculature (i.e. Acetazolamide, CO2, head down tilt, microgravity). Our multi-shell protocol provides improved quantitative markers to model the extent of CSF and free water contamination, and might be used to better understand both normal and pathological tissue microstructure. This paradigm is being explored to detect neurodegeneration and neuroinflammation in the presence of edema at high spatial resolution.Acknowledgements
Funded in part by the DUNN Research FoundationReferences
1. Le Bihan D, Breton E, Lallemand D, et al. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401-7.
2. Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103(3):247-54.
3. Jensen JH, Helpern JA, Ramani A, et al. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging Magn Reson Med. 2005;53:1432-40.
4. Alexander AL, Hasan KM, Lazar M, et al. Analysis of partial volume effects in diffusion-tensor MRI. Magn Reson Med. 2001;45:770-80.
5. Hasan KM, Arfanakis K, Alexander AL. A referenceless, balanced and efficient encoding scheme for diffusion tensor imaging. In: Proc 10th Annual Meeting ISMRM, Honolulu, 2002.
6. Fieremans E, Jensen JH, Helpern JA. White matter characterization with diffusional kurtosis imaging. Neuroimage. 2011;58:177-88.
7. Hasan KM. A framework for quality control and parameter optimization in diffusion tensor imaging: theoretical analysis and validation Magn Reson Imaging. 2007;25:1196-202.
8. Hasan KM, Parker DL, Alexander AL. Comparison of gradient encoding schemes for diffusion-tensor MRI. J Magn Reson Imaging. 2001;13:769-80.
9. Hasan KM, Narayana PA. Computation of the fractional anisotropy and mean diffusivity maps without tensor decoding and diagonalization: Theoretical analysis and validation. Magn Reson Med. 2003;50:589-9.
10. Hoy AR, Koay CG, Kecskemeti SR, Alexander AL. Optimization of a free water elimination two-compartment model for diffusion tensor imaging. Neuroimage. 2014;103:323-3.
11. Veraart J, Rajan J, Peeters RR, et al. Comprehensive framework for accurate diffusion MRI parameter estimation. Magn Reson Med. 2013;70:972-84.
12. Tax CM, Otte WM, Viergever MA, et al. REKINDLE: robust extraction of kurtosis INDices with linear estimation. Magn Reson Med. 2015;73:794-808.
13. Hasan KM, Walimuni IS, Abid H, Hahn KR. A review of diffusion tensor magnetic resonance imaging computational methods and software tools. Comput Biol Med. 2011;41:1062-72.
14. Concha L, Gross DW, Beaulieu C. Diffusion tensor tractography of the limbic system.AJNR Am J Neuroradiol. 2005;26(9):2267-74.
15. Hoy AR, Kecskemeti SR, Alexander AL. Free water elimination diffusion tractography: A comparison with conventional and fluid-attenuated inversion recovery, diffusion tensor imaging acquisitions. J Magn Reson Imaging. 2015;42(6):1572-81.
Figures