1671
Structural and functional brain connectivity highlights in neurosensorial profound deafness1Department of Physics, FFCLRP, University of São Paulo, Ribeirão Preto, Brazil, 2Department of Computing and Mathematics, FFCLRP, University of São Paulo, Ribeirão Preto, Brazil, 3Department of Medical Clinics, FMRP, University of São Paulo, Ribeirão Preto, Brazil
Synopsis
The absence of auditory stimuli for a long period leads to modifications in brain structural and functional connectivity. However, the relationship between the brain changes and neurosensorial hearing loss is not fully clarified. In this study we considered a group of subjects with pre-lingual congenital deafness and analyzed their structural and functional connectivity. Our results suggest that auditory input deprivation not only alters the activity of sensory areas but also reshape the structural and functional organization of cognitive-related networks. These findings can be instructive to clinical practice.
Introduction and Purpose
Neurosensorial hearing loss (NHL) is a type of mild, moderate, severe, or total hearing loss that is due to alterations in the vestibulocochlear nerve, inner ear, or central processing centers of the brain1. Although there are many hypothesized pathologies no cause is identified in most cases. Clinical assessment, audiometric testing, and MRI of brain and internal auditory canal are useful in confirming a diagnosis of NHL, evaluating hearing loss severity, and planning treatment2. The absence of auditory stimuli for a long period leads to modifications in structural and functional connectivity3. However, the relationship between the brain changes and NHL is not clarified. If we know which brain functions are altered in people with NHL, we can design effective early rehabilitation training protocols to maintain brain functions.Materials and Methods
Eighteen right-handed subjects with pre-lingual congenital deafness (comprehending those whose presented a bilateral profound hearing loss (>90 dB) up to three years old), aged 18 to 45, of both sexes, with at least 10 years of scholar level, and fluent in Brazilian Sign Language (LIBRAS) were considered. Any subject who presents any neurological, psychological, cognitive or perceived cognitive deficit was not included. This group was matched in both sex and age using a retrospective image research database provided by the Center of Image Sciences and Medical Physics (CCIFM-FMRP). The study was approved by the Ethics in Research Committee.
Imaging acquisition was performed on a 3T Philips system using 32-channel head coil. For anatomical reference, images were acquired using a 3D T1-weighted GE sequence (TR/TE=7/3.1 ms, FA=8°, FOV=240x240 mm2, 160 1-mm slices). Diffusion tensor imaging (DTI) was acquired following the clinical standards: EPI, 32 gradient directions covering the whole sphere, 72 axial images with 2mm isotropic resolution, FOV=256x256 mm, TR/TE=8391/65 ms, b-factor=1000 mm/s2. BOLD-fMRI images were acquired at resting-state using EPI, TR/TE=2000/30 ms, FA=90°, 31 4-mm slices, gap=0.5 mm, 320 repetitions.
DTI was pre-processed for motion and eddy correction (FSL-EDDY4), data outlier removal using spherical-harmonics interpolation assuming 2 fibers; structural connectivity matrix reconstruct by probabilistic tractography framework (FMRIB-FSL5). For BOLD-fMRI, SPM126 and CONN7 toolbox were used. Functional connectivity (FC) template of 32 networks7 was applied in CONN software, where the Pearson correlation between the mean time series of each region was calculated (p-FDR<0.01). Furthermore, the same FC template was applied on the structural connectivity (SC) analysis, resulting in a structural analysis of the corresponding FC networks.
Results
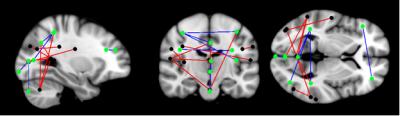
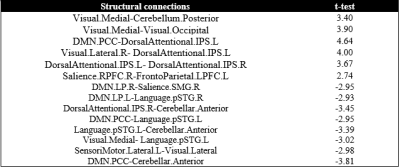
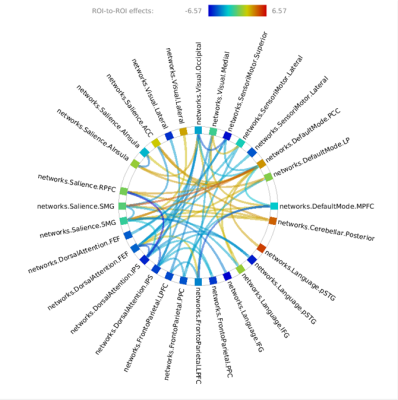
Patient group showed widespread disruption in SC (Figure 1, Table 2) and FC (Figure 2, Table 3) when compared to control group. In general, the pattern disruption correlated with previous findings, with structural and functional reorganization of the brain including plasticity in the sensory cortex (visual occipital and sensorimotor networks) and changes in cognitive processing (dorsal attention (DAN), salience, language and default mode (DMN) networks).Discussions
Results showed impaired SC in cerebellar network and its interactions. It is known that motor cortex and cerebellum are thought to be critical for sensorimotor learning in speech8. Previously reported, people with hearing impairments have balance and motor deficits due to concomitant damage to vestibular and cerebellar structures9. Additionally, cerebellar regions coupled with DAN are recruited in visual working memory and attentional paradigms, while the coupled with DMN are suppressed during task performance10. This antagonistic relationship between the DAN and DMN extends to the cerebellum providing evidence for the active participation of cerebellar nodes in brain function. This functional finding correlates with structural findings in our study. Finally, visual and language SC disruption suggests that the use of sign language tracks important connection in deaf.
Deaf presented decreased FC when compared to controls between bilateral Salience.SMG and DMN.PCC. Salience and DMN are important for cognitive control11. The absence in perception of auditory stimuli can change the alertness of salience and its relationship with DMN. Left language networks interactions suffered reduction in patient groups, while right language networks interactions increased. It is known that language is lateralized in the left hemisphere12. This change is expected due to the different development of language in this group. Finally, the DMN.MPFC and FPN FC disruption is important: MPFC is part of the dorsal pre-frontal cortex and modulate social cognition and behavior, being involved in memory and decision making13.
Conclusion
Our results suggest that auditory input deprivation not only alters the activity of sensory areas but also reshapes the structural and functional organization of cognitive-related networks. Studying the SC and FC in patients with NHL will help us understand the changes in brain structure and function that occur after acoustic input deprivation, which could be instructive to clinical practice.Acknowledgements
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, CAPES, Brasil.References
1. Verbist, Berit M. “Imaging of Sensorineural Hearing Loss: A Pattern-Based Approach to Diseases of the Inner Ear and Cerebellopontine Angle.” Insights into Imaging 3.2 (2012): 139–153. PMC.
2. Lyness, Rebecca C. et al. “Microstructural Differences in the Thalamus and Thalamic Radiations in the Congenitally Deaf.” Neuroimage 100.100 (2014): 347–357. PMC.
3. Butler, Blake E., and Stephen G. Lomber. “Functional and Structural Changes throughout the Auditory System Following Congenital and Early-Onset Deafness: Implications for Hearing Restoration.” Frontiers in Systems Neuroscience 7 (2013): 92. PMC.
4. Jesper L. R. Andersson and Stamatios N. Sotiropoulos. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage, 125:1063-1078, 2016.
5. M. Jenkinson, C.F. Beckmann, T.E. Behrens, M.W. Woolrich, S.M. Smith. FSL. NeuroImage, 62:782-90, 2012
6. Wellcome Department of Imaging Neuroscience, University College, London, UK; www.fil.ion.ucl.ac.uk/spm/
7. Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn : A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. , v. 2, n. 3, 2012.
8. Daniel R. Lametti, Harriet J. Smith, Phoebe Freidin, Kate E. Watkins. Cortico-Cerebellar Networks Drive Sensorimotor Learning in Speech. bioRxiv 177527; doi: https://doi.org/10.1101/177527.
9. Mahdi Majlesi, Nader Farahpour, Elaheh Azadian, Mahdi Amini, The effect of interventional proprioceptive training on static balance and gait in deaf children, In Research in Developmental Disabilities, Volume 35, Issue 12, 2014, Pages 3562-3567, ISSN 0891-4222.
10. Brissenden, James A. et al. “Functional Evidence for a Cerebellar Node of the Dorsal Attention Network.” The Journal of Neuroscience 36.22 (2016): 6083–6096. PMC.
11. Putcha, Deepti et al. “Salience and Default Mode Network Coupling Predicts Cognition in Aging and Parkinson’s Disease.” Journal of the International Neuropsychological Society : JINS 22.2 (2016): 205–215. PMC.
12. Gutierrez-Sigut, Eva et al. “Language Lateralization of Hearing Native Signers: A Functional Transcranial Doppler Sonography (fTCD) Study of Speech and Sign Production.” Brain and language 151 (2015): 23–34. PMC.
13. McKlveen, Jessica M., Brent Myers, and James P. Herman. “The Medial Prefrontal Cortex: Coordinator of Autonomic, Neuroendocrine, and Behavioral Responses to Stress.” Journal of neuroendocrinology 27.6 (2015): 446–456. PMC.
Figures