Petr Bednarik1, Alena Svatkova2, Silvia Mangia1, Christophe Lenglet1, Antoinette Moran2, and Amir Moheet3
1Radiology, Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Department of Pediatrics, University of Minnesota, Minneapolis, MN, United States, 3Department of Medicine, University of Minnesota, Minneapolis, MN, United States
Synopsis
Cystic fibrosis (CF) is the most common fatal
autosomal recessive disorder in Caucasians. As the effects of CF on the brain
structure remain unexplored, we piloted initial MRI investigations of brain
structure by diffusion weighted imaging in CF and cystic fibrosis related diabetes
(CFRD), a common complication in CF patients. Diffusion metrics were obtained in
selected white and gray matter regions of 5 healthy controls (HC) and 5 CF patients
with CFRD. Diffusion metrics of deep gray matter structures appeared to differ between
patients with CF and HC, possibly related to increased iron deposition,
warranting more comprehensive MRI investigations in larger cohorts of patients.
Introduction
Cystic fibrosis (CF) is the most common fatal
autosomal recessive disorder in Caucasians. It is caused by mutations in the gene
encoding the cystic fibrosis transmembrane conductance regulator (CFTR), an anion
channel that conducts bicarbonate and chloride (Cl-) across cell
membranes. Although defective anion transport across epithelial cells is
accepted as the basic defect in CF, the CFTR is also widely expressed in the
central and peripheral nervous system (NS).1 While traditionally NS is not viewed as an organ
impacted in CF, several NS abnormalities have been described in CF.¹ Cystic
fibrosis related diabetes (CRFD) is a common complication in patients with CF.
CF is also associated with chronic systemic inflammation.² Both diabetes and
chronic inflammation have also been postulated to affect brain structure and
function. Structural and functional abnormalities of peripheral nerves were
observed in the porcine model of the CF.2 Effects of CF and CF related risk factors on
human brain structure remain unexplored. In this study we piloted the first MRI
investigation of brain structure of subjects with CF and CFRD by using diffusion
tensor imaging (DTI).Methods
Five patients with CF and CFRD (36±5 y.o, 2
males, BMI 23.3±3.6 kg/m2) were
age, gender and BMI matched to 5 healthy controls (HC) (34±5 y.o, 2 males, BMI=22.8±3.7
kg/m2). Studies were performed on a 3T Siemens Prisma system using
a 32-channel RF receive coil. T1-weighted (T1w) and T2FLAIR-weighted (FLAIR) images
were collected with MPRAGE and SPACE pulse sequences, respectively, both with
isotropic resolution of (1 mm)3. Two DTI datasets were acquired with
different phase encoding (i.e. anterior-posterior and posterior-anterior) utilizing
TR=2820 ms, TE=72.6 ms; multi band (MB)=4, 7 non-diffusion weighed (b0)
images, and 93 diffusion weighted images with b-value of 750 s/mm2
(47 images) and 1500 s/mm2 (46 images) and voxel size of (1.8 mm)3.
Brain segmentation of the T1w scan was carried out with FreeSurfer (FS). FLAIR
was used to refine delineation of cortical-pial surface in FS. A trained
operator visually inspected each subject’s data to ensure accuracy of the
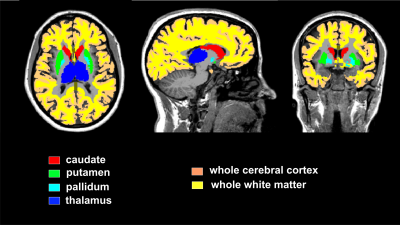
segmentation. The following region of interest (ROI) masks were derived from FS
automatic labeling of each subject’s brain anatomy (Fig. 1): whole supratentorial
white matter, whole cortical grey matter, and large deep brain grey matter
structures including thalamus, putamen, pallidum and caudate. The masked ROIs
extracted from the left and right hemispheres were combined before averaging. DTI
datasets with opposite phase encoding were utilized to reduce susceptibility
artifacts and field inhomogeneities using FSL TOPUP. After motion and
eddy-current correction, DTI data were skull-stripped and BB-registered to the
T1w. The DTIFIT tensor model in FSL was fit to generate fractional anisotropy
(FA), mean (MD), axonal (AD) and radial diffusivity (RD) maps. The values of
the respective DTI parameters were averaged across voxels within each ROI per subject
and compared with two-tailed t-test between groups. Bonferroni correction was
used for multiple testing of the 6 ROIs.Results and Discussion
T1w and FLAIR contrast did not reveal signal-abnormalities as assessed
visually by a radiologist. The comparison of diffusion metrics between healthy
controls and CF patients revealed increases related to CF of FA by 10.7% (p=0.048, corrected) and AD by 5.5% (p=0.018,
corrected) in putamen. A trend for higher RD
by 7.5% was also observed in pallidum (p=0.054,
corrected). Such changes suggest higher iron deposition in those subcortical
structures, which can be physiologically observed in elderly due to aging.3,4 Deep gray matter was not assessed in
previous DTI studies in type 1 diabetes5 and therefore, we cannot rule out that
the co-occurring diabetes contributes to occurrence of this pattern resembling
“accelerated aging”. In addition, due to malabsorption of fat soluble vitamins,
CF is often associated with vitamin E and K deficiency, which can be another
mechanism of increased iron deposition in CF.6 In our sample, it is unclear if
polyvalent pharmacotherapy underlies/contributes to our outcomes. No
differences of diffusion metrics were observed in the other ROIs.Conclusios
This pilot DTI investigation of patients with CF
and CFRD revealed changes in diffusion metrics of deep grey matter structures
(putamen and pallidum). Further studies with greater sample size and more sensitive
structural and functional metrics are warranted to elucidate the impact of CF on brain structure and function.Acknowledgements
P30 NS076408, P41 EB015894, Pennsylvania Cystic Fibrosis, Inc.References
[1.] Reznikov, L. R. Cystic Fibrosis and the Nervous Syste]\m. Chest 151, 1147–1155 (2017). [2.] Reznikov, L. R. et al. CFTR-deficient pigs display peripheral nervous system defects at birth. Proc. Natl. Acad. Sci. 110, 3083–3088 (2013). [3.] Pfefferbaum, A., Adalsteinsson, E., Rohlfing, T. & Sullivan, E. V. Diffusion tensor imaging of deep gray matter brain structures: Effects of age and iron concentration. Neurobiol. Aging 31, 482–493 (2010). [4.] Xu, X., Wang, Q., Zhong, J. & Zhang, M. Iron deposition influences the measurement of water diffusion tensor in the human brain: a combined analysis of diffusion and iron-induced phase changes. Neuroradiology 57, 1169–1178 (2015). [5.] Kodl, C. T. et al. Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive function. Diabetes 57, 3083–3089 (2008). [6.] Wongmongkolrit, T., Wyszynski, R., Hershey, C. O. & Varnes, A. W. Evidence of Subclinical Extrapyramidal Hemosiderosis in Cystic Fibrosis. (1985).