1659
Functional organisation of the hyperdirect pathway by in vivo structural connectivity imaging in healthy humans at 3T1Brain and Spine Institute, CNRS UMR 7225 - INSERM U 1127 - UPMC-P6 UMR S 1127, Paris, France, 2Center of NeuroImaging Research - CENIR, Paris, France, 3AP-HP, Hôpital de la Pitié-Salpêtrière, Department of Neurosurgery, Paris, France, 4Surgical Planning Laboratory, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
Synopsis
The goal of this study is to investigate the anatomo-functional organization of the hyperdirect pathway between the subthalamic nucleus (STN) and the cortex in humans. We identified motor, limbic and associative areas of the whole cortex. We used DWI from 30 healthy subjects and probabilistic tractography between the STN and 39 cortical areas. The motor part of the hyperdirect pathway was found predominant compare to the limbic and above all the associative parts.
Introduction
The subthalamic nucleus (STN) is a critical component of normal motor function. It has become the main target for deep brain stimulation (DBS) in Parkinson’s disease patients to significantly improve motor functions in patients with advanced forms of the disease. While the underlying mechanism of action for DBS is still unknown, the potential modulation of white matter tracts connecting the surgical targets has become an active area of research1.
The hyperdirect pathway is a direct excitatory connection from the cortex to the STN. While the motor part of the hyperdirect pathway has been extensively studied2-5, the associative and limbic components are much less understood. Understanding the associative and limbic components of the hyperdirect pathway might help to minimize borders effects observed in some patients during DBS surgery and would improve DBS clinical results.
The aim of this study is to investigate the anatomo-functional organization of the hyperdirect pathway, and to focus on the associative and limbic cortical areas compared to the motor components.
Methods
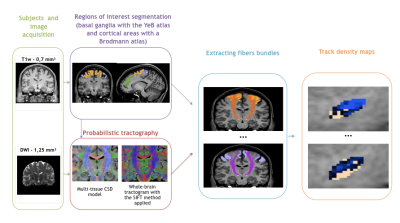
The methodological pipeline is summarised in Fig. 1.
We used DWI acquired at 3T (1.25 mm isovoxel size) from 30 healthy subjects included and preprocessed in the Human Connectome Project (HCP)6.
Data were processed with MRtrix and we estimated the distribution of fiber orientations (FOD) present within each imaging voxel using the Constrained Spherical Deconvolution framework7. Probabilistic whole-brain tractography was run with the following parameters: 100 million tracks, step length of 0.1 mm, curvature threshold of 45°, minimum tract length of 6 mm, maximum tract length of 250 mm and fiber orientation distribution amplitude cut-off of 0.1. The spherical-deconvolution informed filtering of tractograms was applied to reduce the number of streamlines to 10 million to provide a biologically meaningful estimate of structural connection density by removing false positive tracts8.
78 tracts were extracted between 39 cortical areas (segmented using a Brodmann atlas) and the STN (segmented using our in-house 3D histological and deformable atlas of the human basal ganglia9).
Track-density maps were created to observe the cortical projections onto the STN10.
Results
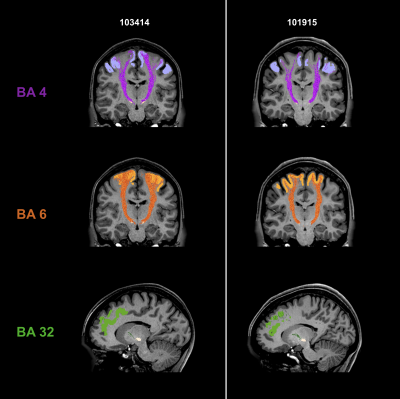
Fig. 2 shows individual tractography reconstructions of three pathways between the STN and Brodmann areas in two subjects. The reconstructed tracts are symmetric and consistent with the expected anatomy4,5. The tracks innervate the whole regions of interest.
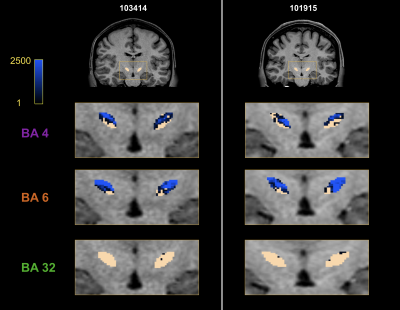
Fig. 3 presents the track-density maps at the STN level for the three previous fibers bundles. We observe a topological organization of the motor connectivity. Connections between the area 6 and STN are slightly more medial than connections between the area 4 and STN.
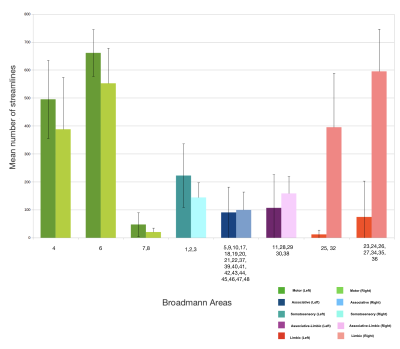
Fig. 4 shows the average number of streamlines across the 30 healthy subjects. Motor areas have the highest number of connections with the STN while limbic areas and above all the associative ones have a lower connectivity strength with the STN. Limbic connectivity is more dominant in the right hemisphere than the left.
Conclusion
Our results on the connectivity between the cortex and STN using DWI data from 30 healthy subjects of the HCP show that the motor part of the hyperdirect pathway is much more predominant than the limbic part, while that the associative part is much more restricted.
It would be interesting to classify STN voxels based on their cortical connectivity densities.
Acknowledgements
The research leading to these results received funding from the programs "Institut des neurosciences translationnelles" ANR-10-IAIHU-06 and "Infrastructure d’avenir en Biologie Santé" ANR-11-INBS-0006.
SBS has been supported by the Bettencourt Schueller Foundation and by the National Agency for Research under the program "Investissements d’avenir" ANR-10-EQPX-15.
References
1. Kringelbach ML, Jenkinson N, Owen SLF, Aziz TZ. Translational principles of deep brain stimulation. Nature reviews. Neuroscience, 2007; 8(8) :623–635.
2. Nambu A, Takada M, Inase M, Tokuno H. Dual somatotopical representations in the primate subthalamic nucleus: evidence for ordered but reversed body-map transformations from the primary motor cortex and the supplementary motor area. J. Neurosci. 1996; 16 (8), 2671–2683
3. Haynes W, Haber S. The organization of prefrontal subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for basal ganglia models and deep brain stimulation. J Neuroscience, 2013; 33:4804–4814.
4. Brunenberg EJL, Moeskops P, Backes WH, Pollo C, Cammoun L, Vilanova A, Janssen MLF, Visser-Vandewalle V, Romeny B, Thiran J, Platel B. Structural and Resting State Functional Connectivity of the Subthalamic Nucleus: Identification of Motor STN Parts and the Hyperdirect Pathway. Plos One. 2007.
5. Petersen MV, Lund TE, Sunde N, Frandsen J, Rosendal F, Juul N, Ostergaad K. Probabilistic versus deterministic tractography for delineation of the cortico-subthalamic hyperdirect pathway in patients with Parkinson disease selected for deep brain stimulation. J Neurosurg. 2016; 8:1–12.
6. Essen V, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K. The WU-Minn Human Connectome Project : An overview. NeuroImage. 2013; 80:62-79
7. Dhollander T, Raffelt D, Connelly A. Unsupervised 3-tissue response function estimation from single-shell or multi-shell diffusion MR data without a co-registered T1 image. ISMRM Workshop on Breaking the Barriers of Diffusion MRI, 2016, 5.
8. Smith RE, Tournier JD, Calamante E, Connelly A. SIFT: Spherical-deconvolution informed filtering of tractograms. NeuroImage. 2013;67:298-312.
9. Bardinet E, Bhattacharjee M, Dormont D, Pidoux B, Malandain G, Schüpbach M, Ayache N, Cornu P, Agid Y, Yelnik J. A three-dimensional histological atlas of the human basal ganglia. II. Atlas deformation strategy and evaluation in deep brain stimulation for Parkinson disease. J. Neurosurg. 2009;110, 208–219.
10. Calamante F, Tournier JD, Jackson GD, Connelly A. Track-density imaging (TDI): Super-resolution white matter imaging using whole-brain track-density mapping. NeuroImage. 2010;53, 1233-1243
Figures