1658
Altered white matter tracts in schizophrenia with persistent negative symptoms1Institute of Medical Device and Imaging, National Taiwan University College of Medicine, Taipei, Taiwan, 2Department of Radiology, Wei Gong Memorial Hospital, Miaoli, Taiwan, 3Department of Psychiatry, National Taiwan University Hospital, Taipei, Taiwan, 4Graduate Institute of Brain and Mind Sciences, National Taiwan University College of Medicine, Taipei, Taiwan, 5Department of Medical Imaging, National Taiwan University Hospital, Taipei, Taiwan, 6Molecular Imaging Center, National Taiwan University, Taipei, Taiwan
Synopsis
This article aimed to investigate the alteration of white matter tracts in schizophrenia with persistent negative symptoms (PNS) in an attempt to identify white matter tracts that are characteristic of PNS. We performed diffusion spectrum imaging (DSI) and whole brain tract-based automatic analysis (TBAA) to compare the tract integrity among healthy controls,
Introduction
Negative symptoms of schizophrenia are associated with poor outcomes [1], and a reduced likelihood of remission [2,3]. In first episode schizophrenia, approximately 27 percent of patients suffer from persistent negative symptoms (PNS), the symptoms that are largely resistant to treatment [4]. PNS has been considered to associate with characteristic change in brain structure and function [5]. Previous studies explored white matter integrity in PNS using a region-of-interest approach. The results showed no significant differences between PNS and non-PNS groups [4]. The present study recruited a relatively large number of patients and conducted a tract-based automatic analysis (TBAA) [6] over the whole brain. By comparing the tract integrity among healthy controls, PNS and non-PNS groups, we aimed to identify altered white matter tracts that were characteristic of PNS.Methods
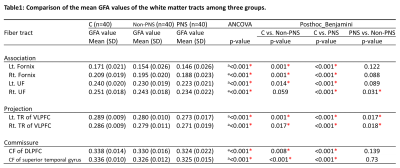
Eighty chronic patients with schizophrenia and 40 healthy controls (male = 20, age = 30.83 ± 8.66 years) were recruited in the study. Patients were divided into PNS (n = 40, male = 20, age = 30.77 ± 9.63 years) and non-PNS (n = 40, male = 20, age = 30.36 ± 8.86 years) groups. Patients were assessed using the PANSS scores after 6 months of treatment [7]. Patients with total score of negative items (N1+N2+N3+N4+N6) >=15 were considered to have PNS [8]. All subjects were scanned on a 3T MRI system (TIM Trio, Siemens, Germany). DSI was performed using 102 diffusion encoding gradients with bmax = 4000 s/mm2 (TR/TE = 9600/130 ms, image matrix size = 80 x 80, spatial resolution = 2.5 mm x 2.5 mm, and slice thickness = 2.5 mm) [9]. TBAA was used to analyze tract integrity over the whole brain [6]. The output of TBAA was a 2D connectogram for each DSI dataset, presenting generalized fractional anisotropy (GFA) profiles of the 76 tract bundles. Connectograms of GFA from the PNS, non-PNS and control groups were compared using one way ANCOVA analysis with age and sex as the covariates. The Benjamini-Hochberg method was used to correct for multiple comparisons.Results
Group differences in white matter tract integrity were observed in the bilateral fornices, bilateral uncinate fasciculi (UF), bilateral thalamic radiations (TR) of the ventral lateral prefrontal cortex (VLPFC), callosal fiber (CF) connecting bilateral dorsal lateral prefrontal cortices (DLPFC) and CF connecting bilateral superior temporal gyri (Table 1). These tracts showed a gradation of GFA decrease from healthy controls, non-PNS to PNS patients. As compared with the control group, post-hoc analysis showed that the PNS group had significantly lower GFA in all 8 tracts, and the non-PNS group had significantly lower GFA values than the control group in 7 tracts (except the right UF). The PNS group had lower GFA than the non-PNS group in 3 tracts, namely the right UF and bilateral TR of the VLPFC.Discussion
In this study, we found 8 tracts that were significantly different among healthy controls, PNS and non-PNS patients. Post hoc analysis showed that the right UF exhibited tract alteration only in PNS patients. The UF is a major fiber tract bundle that connects the orbitofrontal and anterior temporal lobes[10]. Disruption in the frontal and temporal lobes has been implicated in negative symptoms of schizophrenia [11]. We also observed altered bilateral TR of the VLPFC in both PNS and non-PNS. This finding suggests that TR of the VLPFC alters along with the severity of negative symptoms. The thalamus is a part of the frontal, limbic, and cerebellar circuits and plays an important role in the regulation of cognition, affect, emotion and behavior [12]. The size of the thalamus has been found to associate with poorer clinical outcomes [13] and persistent negative symptoms [14]. In conclusion, our results converge with previous findings and suggest that the alteration of white matter tract integrity in the right UF and bilateral TR of the VLPFC may be characteristic of PNS.Acknowledgements
No acknowledgement found.References
1. Patel, Rashmi, et al. "Negative symptoms in schizophrenia: a study in a large clinical sample of patients using a novel automated method." BMJ open 5.9 (2015): e007619.
2. Kirkpatrick B, Fenton WS, Carpenter WT et al. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull 2006;32:214–19.
3. Moller HJ, Bottlender R, Wegner U et al. Long-term course of schizophrenic, affective and schizoaffective psychosis: focus on negative symptoms and their impact on global indicators of outcome. Acta Psychiatr Scand Suppl 2000;407:54–7.
4. Hovington,C.L.,Bodnar,M.,Joober,R.,Malla,A.K.,Lepage,M.,2012.Identifying persistent negative symptoms in first episode psychosis.BmcPsychiatry12, 224.
5. Sarkar, Sonali, Kiley Hillner, and Dawn I. Velligan. "Conceptualization and treatment of negative symptoms in schizophrenia." World journal of psychiatry 5.4 (2015): 352.
6. Chen, Y.J., Lo, Y.C., Hsu, Y.C., Fan, C.C., Hwang, T.J., Liu, C.M., Chien, Y.L., Hsieh, M.H., Liu, C.C., Hwu, H.G., Tseng, W.Y. (2015) Automatic whole brain tract-based analysis using redefined tracts in a diffusion spectrum imaging template and an accurate registration strategy. Hum Brain Mapp, 36:3441-58.
7. Andreasen, N.C., Carpenter Jr, W.T., Kane, J.M., Lasser, R.A., Marder, S.R., Weinberger, D.R. (2005) Remission in schizophrenia: proposed criteria and rationale for consensus. 2005, 162:441-449
8. Buchanan, R. W. (2006). Persistent negative symptoms in schizophrenia: an overview. Schizophrenia bulletin, 33(4), 1013-1022.
9. Kuo, L. W., Chen, J. H., Wedeen, V. J., & Tseng, W. Y. I. (2008). Optimization of diffusion spectrum imaging and q-ball imaging on clinical MRI system. Neuroimage, 41(1), 7-18.
10. Ungerleider LG, Gaffan D, Pelak VS. Projections from inferior temporal cortex to prefrontal cortex via the uncinate fascicle in rhesus monkeys. Exp Brain Res. 1989; 76:473–484.
11. Kitis O, Ozalay O, Zengin B, Haznedaroglu D, Eker MC, Yalvac D, Oguz K, Coburn K, et al. 2012 Reduced left uncinate fasciculus fractional anisotropy in deficit schizophrenia but not in nondeficit schizophrenia. Psychiatry Clin. Neurosci. 66 34–43.
12. Jones, E. G. (2012). The thalamus. Springer Science & Business Media.
13. Brickman, A. M., Buchsbaum, M. S., Shihabuddin, L., Byne, W., Newmark, R. E., Brand, J., ... & Hazlett, E. A. (2004). Thalamus size and outcome in schizophrenia. Schizophrenia research, 71(2), 473-484.
14. Yoshihara, Y., Sugihara, G., Matsumoto, H., Suckling, J., Nishimura, K., Toyoda, T., ... & Sakahara, H. (2008). Voxel-based structural magnetic resonance imaging (MRI) study of patients with early onset schizophrenia. Annals of General psychiatry, 7(1), 25.
Figures