1634
High Resolution Reconstruction of Diffusion Weighted Imaging Using EPI-Corrected Snapshots Acquired with Rotated K-spaces1Harvard Medical School, Boston, MA, United States
Synopsis
We propose a non-Cartesian high resolution reconstruction of diffusion-weighted magnetic resonance imaging (DW-MRI) using multi-snapshots acquired with rotated K-spaces. Our technique boosts the signal level by reducing the echo time and by increasing voxel size for each snapshot. The final high resolution image is reconstructed by fusion of the snapshots, which were corrected for Echo-Planar-Imaging (EPI) distortions. We applied and evaluated different EPI correction methods. Through qualitative and quantitative evaluations based on in-vivo experiments, we showed that our protocol and image reconstruction technique leads to high spatial resolution and high signal-to-noise ratio DW-MRI.
PURPOSE
Our aim was to develop an image acquisition protocol to reconstruct high SNR and high spatial resolution DW-MRI (0.8mm isotropic resolution) using clinical 3T MRI scanners. To do so, we acquired low resolution snapshots (1.6x0.8x1.6 mm) with rotated k-spaces. The final high resolution image was constructed by fusing information of the multi-snapshots, which were corrected individually for the geometric Echo-Planar-Imaging (EPI) distortion.METHOD
We imaged healthy volunteers on a 3T Siemens scanner with a 64 channel head coil. We acquired axial DW-MRI scans at four snapshots corresponding to { 0, pi/4, pi/4, pi/2} rotations of K-space at 20 gradient directions with Anterior-to-Posterior (AP) phase encoding; The parameters were: bvalue=1000s/mm2; Matrix size=128x256x84; resolution=1.6x0.8x1.6mm3; TE=122ms. B0 images with Posterior-to-Anterior (PA) phase encoding directions and double gradient echos of resolution 1.5x1.5x1.5mm3 were also acquired to perform EPI distortion correction.
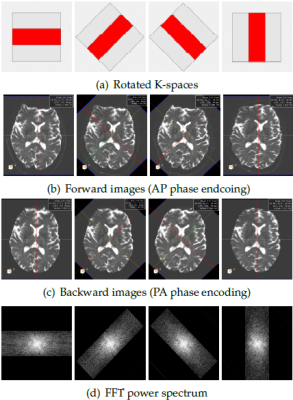
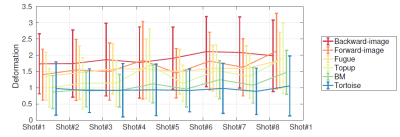
Examples of the acquired low resolution B0 snapshots using rotated K-spaces is shown in Figure.1. Each of these snapshots encodes different high frequency information of the underlying brain structures in the direction of the frequency encoding. Furthermore, it can be seen that different shots exhibit variant geometric distortions due to the changes in the direction of phase encoding. Therefore, EPI correction is a critical step in our framework to ensure that our model-based reconstruction fuses information from the exact same location in the brain. We applied different EPI distortion correction technicques including: Topup [1], the block-matching registration approach [2], Tortoise [3], and FUGUE tool (using the field map) [4]. We assessed the quality of the corrected images by comparing them to the structural image, which we considered as a reference image without distortion. We performed non-rigid registration using ANTS [5] between the corrected B0s and the T2 image to evaluate the amplitude of the remaining distortion. Our hypothesis was that a more successful EPI-correction leads to less remaining deformation.
We considered the following general forward model to generate high resolution image [6]:
yk=Dk Bk SK Mk X + ε (1)
where X represents the unknown high resolution image and yk is its corresponding low resolution snapshot scanned at kth rotation of the K-space; Dk represents downsampling matrix; Bk is a blur matrix modeling the point spread function of the signal acquisition; we model Bk as a sinc function; Sk is modeling the slice selection profile; and Mk is motion parameters considered as rigid transformation in our work (3 rotations and 3 translations). In our implementation, we estimate the high resolution X image by solving the inverse problem of Eq(1) from the observed low resolution yk snapshots.
RESULTS
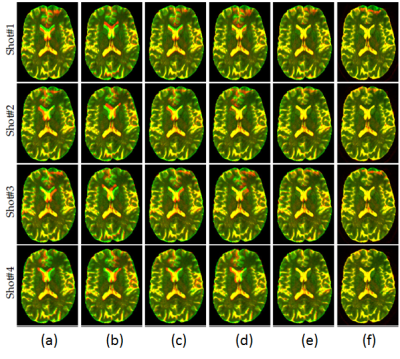
Our qualitative and quantitative validations of EPI corrected data (Figure.2 and Figure.3) indicates that Tortoise leads to the best correction results. Therefore, we used Tortoise in our framework to compensate for EPI-distortion prior high resolution reconstruction.
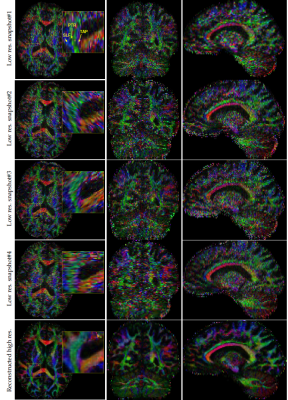
Figure.4 shows the color-FA of the low resolution and the reconstructed high resolution images at three orthogonal planes. A better delineation of the fine structures can be seen in the reconstructed high resolution image.
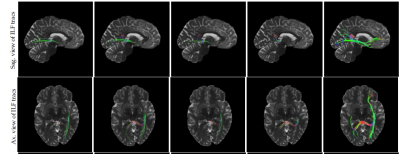
Tractography results using fibernavigator tool with same seeding region and the same tractography parameters for all the scans are shown in Figure.5. It can be seen that the results achieved by high resolution image outperforms the snapshots with low resolution.
DISCUSSION and CONCLUSION
We developed an image acquisition protocol to reconstruct high resolution DW-MRI of 0.8mm isotropic resolution using clinical MRI scanners. Comparing to the most of the existing methods on high resolution DW-MRI [7], which are using snapshots with high in-plane resolution and low resolution in the slice direction, we acquire snapshots with low resolution in the phase encoding and slice directions to boost the signal level, which provides an SNR advantage due to the larger voxel size.
For in-vivo images, we showed that tractography results of our reconstructed images are superior compared to the low resolution snapshots.
The acquired snapshots exhibit space variant geometric distortions due to the changes in the direction of phase encoding. Therefore, to ensure that the model-based reconstruction fuses information from the exact same location in the brain, we integrated EPI distortion correction step into our scheme. Our qualitative and quantitative results for the EPI corrected data indicates that the symmetric diffeomorphic registration based technique (Tortoise) leads to satisfactory distortion compensation in reconstruction.
Note that, in our image acquisition protocol, the effective bandwidth in phase encoding direction was constant without considering factors like integrated parallel imaging techniques (iPAT). One possibility to reduce the geometric distortion effect is increasing iPAT factor. However, it also reduces the signal, which is not desirable. As a part of our future work, we will study the effect of these factors in our scheme.
Acknowledgements
This study was in part supported by the National Institutes of Health (NIH) grants R01 EB019483, R01 EB018988, R01 NS07978. R0 EB013248, and R01 NS079788.References
[1] Andersson, J., Skare, S., Ashburner, J.: How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20(2003) 870–888.
[2] Hedouin, R., Commowick, O., Bannier, E., Scherrer, B., Taquet, M., Warfield, S., Barillot, C.: Block-matching distortion correction of echo-planar images with opposite phase encoding directions. IEEE TMI (2017) 1–10.
[3] Irfanoglu, M., Modi, P., Nayak, A., Hutchinson, E., Sarlls, J., Pierpaoli, C.: Dr-buddi (diffeomorphic registration for blip-up blip-down diffusion imaging) method for correcting echo planar imaging distortions. Neuroimage 20(2015) 284–299.
[4] Jezzard, P., Balaban, R.: Correction for geometric distortion in echoplanar images from b0 field variations. MRM34 (1995) 65–7.
[5] Avants, B., Tustison, N., Johnson, H.: Advanced normalizationtools. 2014.
[6] Gholipour, A., Estroff, J., Warfield, S.: Robust super-resolution volume reconstruction from slice acquisitions: Application to fetal brain mri. IEEE TMI 29 (2010) 1739–1759.
[7] Scherrer, B., Afacan, O., Taquet, M., Prabhu, S., Gholipour,A., Warfield, S.:Accelerated high spatial resolution diffusion-weighted imaging. IPMI (2015).
Figures