1631
Navigated Multi-shot Diffusion-Weighted Imaging with Multiplexed Sensitivity Encoding1Global MR Applications and Workflow, GE Healthcare, Menlo Park, CA, United States, 2Global MR Applications and Workflow, GE Healthcare, Boston, MA, United States, 3Global MR Applications and Workflow, GE Healthcare, Houston, TX, United States, 4Global MR Applications and Workflow, GE Healthcare, Waukesha, WI, United States
Synopsis
MUltiplexed Sensitivity Encoding (MUSE) has been successfully used to correct for motion-induced phase errors in multi-shot diffusion-weighted imaging. However, this technique relies heavily on parallel imaging (PI) and can result in residual aliasing and excessive noise amplification when the number of shots is similar to the number of receiver coil elements. We propose a navigated multi-shot approach with multiplexed sensitivity encoding to handle cases where the coil geometry would otherwise limit the maximum number of interleaves. We show that both PI and 2D-selective excitation pulses can be used to reduce the scan duration, while maintaining similar levels of distortion.
Introduction
MUltiplexed Sensitivity Encoding1 (MUSE) has been successfully used to correct for motion-induced inter-shot phase errors in multi-shot diffusion-weighted imaging (DWI). Despite the better conditioning of the MUSE formulation over conventional parallel imaging (PI), this technique relies heavily on coil sensitivity variations to correct for motion-induced phase errors, making its performance dependent on the specific receiver coil used for signal reception. In addition, especially for body applications, while high-density coils are often used, it is not uncommon to have only two receiver elements in the phase encode direction, which also limits the maximum number of interleaves that can be reconstructed with MUSE without incurring in unwanted residual aliasing or excessive noise amplification. We propose a navigated multi-shot Echo-Planar Imaging (EPI) acquisition with multiplexed sensitivity encoding2 to extend the MUSE approach to cases where the coil geometry or the specific prescription would otherwise limit the maximum number of interleaves. All the advantages of the non-navigated approach in terms of SNR performance and improved matrix conditioning are maintained. In addition, depending on the application, a combination of PI and 2D-selective excitation pulses3 are shown to reduce the overall scan duration, while maintaining similar levels of distortion.Methods
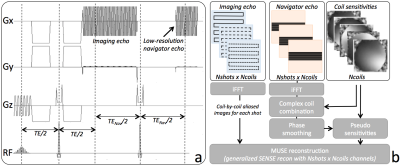
We implemented a twice-refocused, navigated, multi-shot, diffusion-weighted EPI pulse sequence, with a low-resolution, fully sampled 2D navigator acquired after each imaging readout (Figure 1a). Phase correction and ramp sampling correction were performed separately for imaging and navigator data. Complex coil combination of each navigator echo was performed using coil sensitivities estimated from a separate conventional 3D gradient echo scan4. Local unwrapping and smoothing of the navigator phase using a 17x17 boxcar filter was performed to reduce phase inconsistencies in low-SNR regions. For each interleave, the corresponding motion-induced navigator phase was demodulated from the coil sensitivities, yielding Nshots x Ncoils pseudosensitivities. A generalized SENSE reconstruction1 was performed to recover the final phase-corrected, eventually unaliased, imaging volume (Figure 1b). For prostate, a 2D excitation pulse was used to limit the phase encoded FOV to the anatomy of interest. Imaging was performed both at 1.5T (brain - MR450w, GE, Waukesha, WI) and 3T (prostate – MR750, GE, Waukesha, WI), using an 8-channel brain coil and a 32-channel cardiac array for brain and prostate imaging, respectively.Results
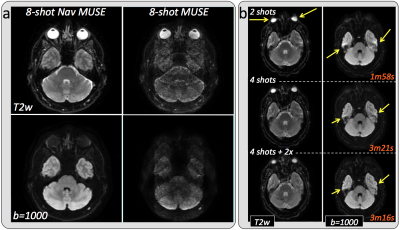
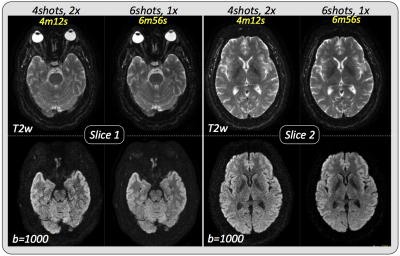
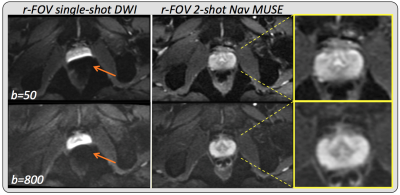
Figure 2a shows a comparison between conventional and navigated MUSE in a healthy volunteer. The non-navigated approach results in unacceptable noise amplification and residual aliasing when the number of interleaves is the same as the number of available receiver coils, while both of these issues are completely resolved when the motion-induced phase estimates are obtained from the navigator data. Scan time scales linearly with the number of interleaves, however, distortion is proportional to the effective phase-encoded FOV, so that the same or even lower levels of distortion can be achieved in significantly less time using PI to synthetize missing samples. As an example, increasing the number of interleaves from 2 to 4 reduces distortion by a factor of 2, while doubling the scan time. Further undersampling a 4-shot acquisition by a factor of 2 results in distortion levels comparable to an 8-shot acquisition, with no additional scan time penalty (Figure 2b). Figure 3 shows two representative slices of a high-resolution brain dataset. In this case, leveraging PI in combination with multi-shot to speed up the acquisition while reducing distortion comes at the cost of visibly reduced SNR. Depending on the specific application, reduced FOV (r-FOV) imaging together with multi-shot can be used instead of PI to effectively reduce distortion without incurring in prohibitively long scan times. Figure 4 shows significant distortion reduction in the prostate of a healthy volunteer using r-FOV 2-shot navigated MUSE compared to conventional r-FOV single-shot. Note that, in this case, because of the axial prescription, there are only two receiver coil elements in the phase encoding direction (anterior/posterior), however, no residual aliasing and adequate SNR were obtained with the proposed navigated approach.Discussion
High resolution DWI suffers from severe distortion and blurring due to the underlying EPI readout. The use of 2D navigators with MUSE effectively decouples the distortion-correction capabilities of this technique from the PI performance of the specific coil used, thus extending its applicability to cases with limited coil sensitivity variations in the phase encoding direction. Nonetheless, PI and, in some cases, r-FOV imaging can be used together with navigated MUSE to reduce the overall scan duration, while maintaining similar levels of distortion.Conclusion
We showed that 2D navigators, PI and r-FOV imaging used in conjunction with MUSE allow high resolution imaging with minimal distortion and clinically acceptable scan times.Acknowledgements
No acknowledgement found.References
1. Chen N, Guidon A, Chang HC, et al. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage 2013; 72:41-47.
2. Sundman MH, Chang HC, Xu D, et al. Enhancing diffusion weighted image (DWI) quality with Navigator-MUSE. In Proceedings of the 23rd Meeting of the ISMRM. Toronto, Canada. 2015, p.2802.
3. Saritas EU, Cunningham CH, Lee JH, et al. DWI of the spinal cord with reduced FOV single-shot EPI. Magn Reson Med. 2008; 60(2):468-473.
4. Roemer PB, Edelstein WA, Hayes CE, et al. The NMR phased array. Mag Res Med. 1990; 16(2):192-225.
Figures