1625
Shorter Acquisition Times for Diffusion-Weighted Imaging of the Human Spinal Cord with Simultaneous Acquisition of Multiple Inner Fields-of-View1Systems Neuroscience, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Synopsis
Inner field-of-view EPI is widely used for diffusion-weighted acquisitions of the human spinal cord. However, due to the high in-plane resolution required acquisition times to achieve a reasonable signal-to-noise ratio are usually rather long. In this study, inner-field-of-view EPI based on 2D-selectove RF excitations is accelerated with multiband excitations. Two different approaches are considered that differ with respect to the orientation of the 2DRF trajectory and whether side excitations must be suppressed or can be used to cover the bands excited and acquired simultaneously. Results obtained in the human brains stem and cervical spinal cord in vivo are presented.
Introduction
Inner-field-of-view imaging techniques, e.g. based on 2D-selective RF (2DRF) excitations [1, 2] have been shown to improve the image quality for diffusion-weighted echo-planar imaging of the human spinal cord in vivo (e.g. [3, 4]). Due to the high spatial resolution desired, acquisition times are typically about 10 minutes or longer which may hamper the applicability in practice. In this study, two different approaches to shorten the acquisition time by the simultaneous acquisition of multiple inner fields-of-view (e.g. [5]) are considered and demonstrated in phantom and in vivo experiments.Methods
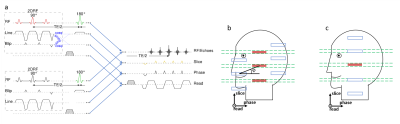
The basic EPI sequences and geometric setups used are presented in Fig. 1. Both sequences involve a 2DRF pulse for the initial excitation, however, they differ regarding (i) its orientation relative to the imaging part and (ii) the desired excitation profile. In the first sequence, the 2DRF trajectory is rotated by an angle ϕ compared to the slice and phase-encoding direction (“tilted”, upper in Fig. 1a) [3]. This allows to position the side excitations occurring in the blip direction between the multiple slices to be refocused (Fig. 1b) which reduces the field-of-excitation and the minimum 2DRF pulse length required but also means that all fields-of-view to be excited need to be included in the desired excitation profile (Fig. 1b). In the second sequence (“swapped”) the blip and line direction of the 2DRF trajectory coincide with the slice and phase directions of the imaging part, respectively (lower in Fig. 1a) [4]. Thus, the side excitations can be positioned to cover the other inner fields-of-view to be acquired, i.e. it is sufficient to define only one rectangle as the desired excitation profile (Fig. 1c).
Experiments were performed on a 3 T whole-body MR system (Magnetom PrismaFit, Siemens, Erlangen, Germany). Phantoms and healthy volunteers were investigated with a 64-channel head-neck coil with volunteers giving their informed consent prior to the examination. 2DRF envelopes were calculated using the low-flip-angle approximation [2] and were designed to excite rectangular profile(s) with a size of 4.0x32 mm2. The resolution of the underlying trajectory was 2.5x10 mm2. Echo-planar images were obtained with a resolution of 1.0x1.0x4.0 mm3 covering a field of view (FOV) of 32 x 128 mm² with 16 mm oversampling in the phase encoding direction to account for profile imperfections. Diffusion weighting was applied in six non-collinear directions with a b-value of 500 s mm-2 yielding echo times between 60 and 70 ms and TRs between 5100 ms and 2600 ms without and with multiband acceleration (acceleration factor 2), respectively. Some acquisitions were performed with blipped-CAIPI [5] gradient pulses to induce an apparent relative shift of 50% of the field-of-view between the slices excited and acquired simultaneously. Non-accelerated acquisition were performed with the “tilted” approach.
Results and Discussion
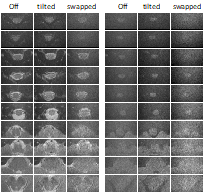
Results of phantom measurements are presented in Fig. 2. All multiband accelerated acquisition provide an image quality similar to the conventional acquisition, in particular, no residual aliasing artifacts are observed. Furthermore, applying blipped-CAIPI did not offer advantages compared to the “unblipped” approach, most likely because for such small shifts no gain in the g factors could be achieved. This is why in vivo acquisitions were performed without blipped-CAIPI.
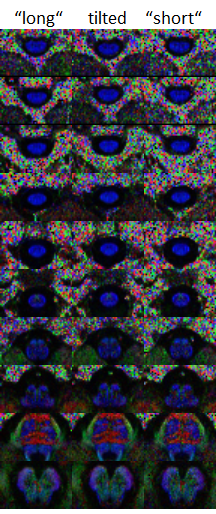
Figure 3 presents non-diffusion-weighted and diffusion-weighted images acquired in the human brain stem and spinal cord in vivo. Most striking is the low performance of the swapped approach with a significantly lower SNR. Presumably, this is a consequence of the low bandwidth of the corresponding 2DRF excitation in the blip direction which coincides with the slice direction for this sequence version. This means that even a minor deviation of the frequency, e.g. due to field inhomogeneities, may result in a significant spatial shift and a corresponding signal loss because of a mismatch with the refocusing RF pulse.
Color-coded FA maps of the slices shown in Fig. 3 are compared in Fig. 4. The multiband accelerated acquisition performs better than the non-accelerated acquisition with the same acquisition time (4.6 min) but seems not to achieve the quality of the full acquisition which however takes more than minutes. Although the number of averages is identical (16), this can be expected because due to the much shorter TR (2.6 s vs 5.1 s), saturation effect are significant for the accelerated acquisition.
Conclusion
With multiband acceleration, the acquisition time of diffusion-weighted inner field-of-view echo-planar imaging of the human spinal could be shortened which could help to improve its applicability in clinical practice.Acknowledgements
This work was supported by a grant from Wings for Life.References
[1] Hardy CJ et al.: Spatial localization in 2 dimensions using NMR designer pulses. J Magn Reson. 1989; 82: 647-654
[2] Pauly J et al.: A k-space analysis of small-tip-angle excitation. J Magn Reson, 1989; 81: 43-56
[3] Finsterbusch J, Improving the performance of diffusion-weighted inner field-of-view echo-planar imaging based on 2D-selective radiofrequency excitations by tilting the excitation plane. J Magn Reson. Imaging 2012; 35: 984-92.
[4] Saritas EU, Cunningham CH, Lee JH, Han ET, Nishimura DG. DWI of the spinal cord with reduced FOV single-shot EPI. Magn Reson Med. 2008; 60: 468-73.
[5] Setsompop K et al.: Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Mag Reson Med. 2012; 67: 1210-1224
Figures