1617
High resolution in vivo diffusion weighted imaging of the human occipital cortex: enabled by 300mT/m gradients and flexible radio-frequency surface coils.1Department of Neurophysics, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 2Center for Cognitive Neuroscience Berlin, Free University Berlin, Berlin, Germany
Synopsis
Information about intracortical fibers and connectivity can potentially be obtained using diffusion weighted imaging (DWI). However, in vivo intracortical DWI requires extraordinarily high spatial resolution. We demonstrate in vivo DWI imaging in the human occipital cortex with an isotropic resolution of 800 μm enabled by a high-performance 300 mT/m gradient system and flexible high-sensitivity RF receive coil optimized for cortical imaging. Robust detection of intracortical features was achieved in a reasonable scanning time. The described setup opens the exciting possibility to study intracortical connectomics in humans in vivo.
Introduction
Intracortical connectivity is key to brain function and is strongly supported by the intracortical radial and tangential fibers. Radial fibers are primary efferent/afferent axons leaving/entering the cortex that connect cortical areas with distant areas. Tangential fibers facilitate local cortico-cortical connections, e.g., include collaterals. Diffusion weighted imaging (DWI) has the potential to provide unique information about intracortical fibers in humans as has been shown in post mortem tissue1–3. However, reliable in vivo mapping of intracortical fibers remains an unsolved challenge4. Human cortex has a thickness of only a few millimetres and a much lower fiber density than white matter. Therefore, intracortical DWI requires submillimeter resolution5, high sensitivity and still moderate diffusion weightings6. Here we combined the high-performance 300 mT/m gradient system with a high-sensitivity flexible RF receive coil7. We demonstrate the feasibility of high quality DWI of occipital cortex with an isotropic resolution of 800 μm within a reasonable scan time.Methods
Three participants (2 females, age 25-28 years) took part in a scanning session on a Connectom scanner (3T, Siemens Healthineers, Erlangen, Germany) equipped with a flexible RF receive coil placed tightly around the participant’s back of the head7 (Fig. 1). The coil consists of 23 loop elements with a diameter of 45 mm providing high sensitivity near the elements, i.e., in the cortex. DWI were acquired with a single-shot 2D echo planar imaging (EPI) sequence8–10: 800 μm isotropic resolution, 60 isotropically distributed diffusion directions, ten b=0 images, two shells with b=800,1800 s/mm2, TE=66 ms, TR=8700 ms, bandwidth BW= 1200 Hz/Px, Field-of-View (FOV) 206 mm x 84.5 mm, matrix 256 x 106, 62 oblique nearly axial slices, Partial Fourier 5/8. A saturation band anterior to the occipital lobe was applied to minimize potential fold-over artefacts due to the limited FOV. Two repetitions, each 20 min long, were acquired. In addition, a series of 23 b=0 images was acquired with both the 23-channel surface flex coil and with the manufacturer’s 32 channel head coil in order to estimate image SNR enhancement provided by the flex coil. Sensitivities of both coils were compared by estimating the temporal Signal-To-Noise (tSNR) ratio across the b=0 images. tSNR was used as a proxy for coil sensitivity, since at the resolution of 800 μm thermal noise is expected to dominate. Data were analysed using the ExploreDTI11 package for diffusion analysis. Images were corrected for participant motion and eddy current induced distortions by co-registration to b=0 images. The diffusion tensors were estimated using both shells and a robust tensor estimation algorithm12.Results and Discussion
Maps of tSNR for b=0 images recorded with both
coils are shown in Fig. 2. The receive sensitivity of the flex coil was higher
than of the head coil in superficial brain regions (up to 20 mm depth from the skull
inner surface; ROI1: averaged tSNR 15±4 for flex coil and 8±2.5 for head coil), but decayed rapidly towards the center of the brain
(ROI2: average tSNR 3.6±1.3 for flex coil and 4.6±1.4 for head coil).
Results of DTI analysis for one representative
participant are shown in Figs. 2 and 3 for axial and coronal slices through the
occipital cortex, respectively. White matter tracts such as the optical
radiation were robustly detected. In addition, in the cortex the direction of the
principal eigenvectors corresponded to the cortical normal in many regions (see
inserts a,b,c in Figs. 3 and 4). This is in line with the expected orientation
of intracortical radial fibers, which dominates the DTI direction in deep
cortical layers1. The high acquisition resolution potentially
allows for cortical-depth specific analysis, as can be seen in Fig. 3a. Future
studies will apply advanced diffusion modelling that more appropriately capture
the complex intracortical fiber distributions than DTI.
Conclusions
We demonstrated that combining a high performance gradient system and high sensitivity receive coil makes in vivo DWI acquisition at 800 μm resolution feasible in a realistic scan time of 40 min. DTI results were in line with dominant radial intracortical fibers in the occipital cortex. This novel hardware setup can be readily combined with advanced acquisition schemes5, further improving the resolution and precision. The setup described here opens the exciting possibility of studying intracortical connectomics in humans in vivo.Acknowledgements
The implementation of diffusion weighted single-shot 2D EPI sequence was received from the University of Minnesota Center for Magnetic Resonance Research. The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013) / ERC grant agreement n° 616905. This project has also received funding from the BMBF (01EW1711A & B) in the framework of ERA-NET NEURON.References
1. Leuze, C. W. U. et al. Layer-specific intracortical connectivity revealed with diffusion MRI. Cereb. Cortex N. Y. N 1991 24, 328–339 (2014).
2. Aggarwal, M., Nauen, D. W., Troncoso, J. C. & Mori, S. Probing region-specific microstructure of human cortical areas using high angular and spatial resolution diffusion MRI. NeuroImage 105, 198–207 (2015).
3. Kleinnijenhuis, M. et al. Detailed laminar characteristics of the human neocortex revealed by NODDI and histology. in 4, (2013).
4. McNab, J. A. et al. Surface based analysis of diffusion orientation for identifying architectonic domains in the in vivo human cortex. NeuroImage 69, 87–100 (2013).
5. Setsompop, K. et al. High-resolution in vivo diffusion imaging of the human brain with generalized slice dithered enhanced resolution: Simultaneous multislice (gSlider-SMS). Magn. Reson. Med. n/a-n/a (2017)
6. Setsompop, K. et al. Pushing the limits of in vivo diffusion MRI for the Human Connectome Project. NeuroImage 80, 220–233 (2013).
7. Kriegl, R. et al. Flexible 23-Channel RF Coil Array for fMRI Studies of the Occipital Lobe at 3T. Proceedings of the Annual Meeting of the Organization for Human Brain Mapping OHBM, Honolulu, USA, (2015).
8. Feinberg, D. A. et al. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PloS One 5, e15710 (2010).
9. Moeller, S. et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn. Reson. Med. 63, 1144–1153 (2010).
10. Xu, J. et al. Evaluation of slice accelerations using multiband echo planar imaging at 3 T. NeuroImage 83, 991–1001 (2013).
11. Leemans, A., Jeurissen, B., Sijbers, J. & Jones, D. K. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. in (2009).
13. Tax, C. M. W., Otte, W. M., Viergever, M. A., Dijkhuizen, R. M. & Leemans, A. REKINDLE: Robust extraction of kurtosis INDices with linear estimation. Magn. Reson. Med. 73, 794–808 (2015).
Figures
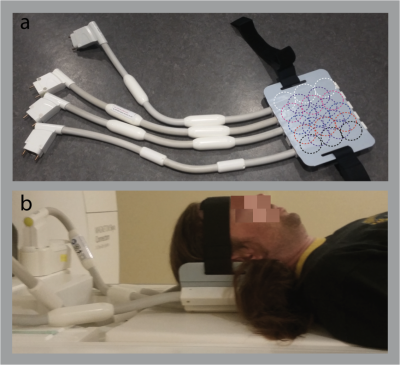
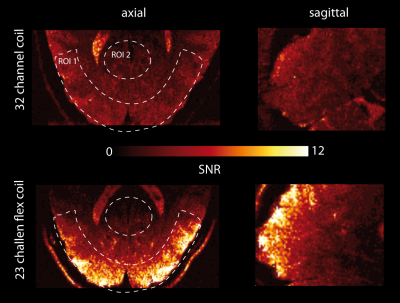
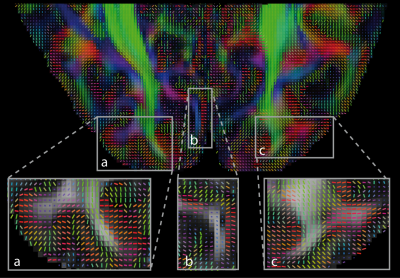
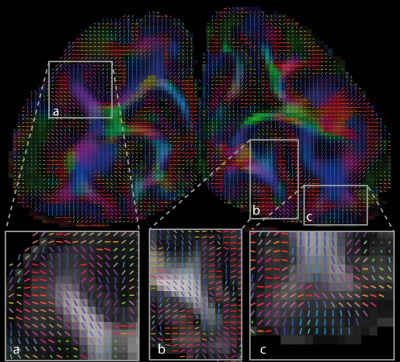
Figure 4. DTI results for an individual. Coronal slice of directionally-encoded colour fractional anisotropy (FA) map overlaid with the tensor glyphs showing the DTI principal eigenvector orientations. High quality estimates of fibre directions were obtained in the cortex (a, b and c) in line with preferential radial orientation of intracortical fibres. High resolution allows studies across cortical depth.