1597
Are Intravoxel Incoherent Motion and Dynamic Contrast-Enhanced Perfusion Parameters Related in Glioblastomas?1Department of Physics, Carleton University, Ottawa, ON, Canada, 2Medical Imaging, The Ottawa Hospital, Ottawa, ON, Canada, 3Radiology, University of Ottawa, Ottawa, ON, Canada, 4Department of Undergraduate Medical Education, University of Ottawa, Ottawa, ON, Canada, 5The Ottawa Hospital Research Institute, Ottawa, ON, Canada
Synopsis
Intravoxel incoherent motion (IVIM) is an MR-based diffusion-weighted imaging technique that can measure both diffusion and perfusion. Currently, no link has been established between the perfusion parameters obtained from IVIM to those from dynamic contrast-enhanced (DCE)-MRI, particularly in the human brain. This study determined that no correlation exists between these two perfusion measurement techniques in patients with glioblastomas. This indicates that these two imaging techniques measure two separate effects; however, IVIM may be able to provide complementary, additional perfusion information that can potentially aid clinical diagnoses when used in conjunction with DCE-MRI parameters.
Introduction
Several methods are used to measure perfusion effects with MRI. In this work, two of these methods will be considered: intravoxel incoherent motion (IVIM) and dynamic contrast-enhanced (DCE)-MRI. It stands to reason that, since they both measure perfusion, a close relationship should exist between the two sets of perfusion parameters. Conversely, if this is not the case, then they characterize different, possibly complimentary, tissue parameters. Currently, there is no reported link between perfusion parameters obtained from IVIM and DCE-MRI. The purpose of this work was to investigate the relationship between these two MR perfusion acquisitions in glioblastomas.
DCE-MRI is a well-established technique for detecting contrast agents as they pass through tissues by relating associated signal changes to tissue perfusion characteristics1. IVIM is a non-contrast diffusion-weighted imaging (DWI) technique that differentiates signal contributions due to diffusion from those due to perfusion2. On a macroscopic scale, blood flow through the microvasculature can be considered incoherent due to the pseudorandom structure of the vessels2; this process is called pseudo-diffusion and manifests as an additional component to the diffusion decay. To observe the IVIM effect, sequentially stronger diffusion gradients differentiate stationary molecules from those undergoing diffusion3. This is modelled by
$$S(b)=S_0[f\cdot e^{-bD^*}+(1-f)\cdot e^{-bD}] \ \ \ (1)$$
where S(b) is the signal-intensity for a given b-value, S0 is the signal-intensity at b=0, f is the perfusion fraction, D is the diffusion coefficient, and D* is the pseudo-diffusion coefficient. Several different methods to evaluate IVIM parameters using Eq. 1 are reported in the literature2,4,5.
Methods
Both IVIM and DCE-MRI were performed pre-operatively on ten patients (median age = 68, 60% male) with glioblastomas. All data was acquired using a 3T MRI system (Magnetom Trio, Siemens) with a 32-channel head coil. For IVIM, DWI images (in trace mode) were acquired using 16 b-values in total (0-900 s/mm2) with 10 values being less than 200 s/mm2. Image data was corrected for both noise and eddy currents. IVIM parameters were evaluated from Eq. 1 using MATLAB and a robust, two-step approach. Parameters f and D were obtained using a mono-exponential function for b≥200 s/mm2. These values were then used with the full bi-exponential fit to determine D*. DCE-MRI was performed using a FLASH sequence with TR=6.5 ms, TE=1.65 ms, and flip angle=30°. DCE-MRI parameters (vp, Ktrans, ve, and kep=Ktrans/ve) were obtained using commercial software. The perfusion parameters from IVIM (f, D*, and their product fD*) were measured for regions of interest (ROIs) on tumour volumes after co-registration to the DCE-MRI data. The ROIs were drawn manually on the DCE-MRI images by a medical student under the supervision of a neuroradiologist. Correlation coefficients between IVIM and DCE-MRI parameters were computed using Spearman’s rank correlation for total tumour tissue (enhancing and necrotic) volumes.Results
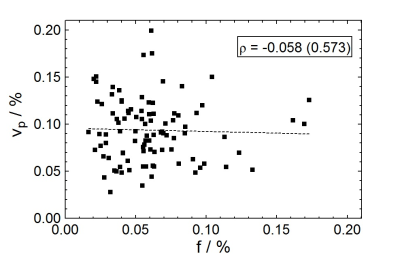
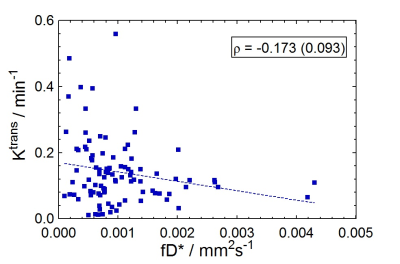
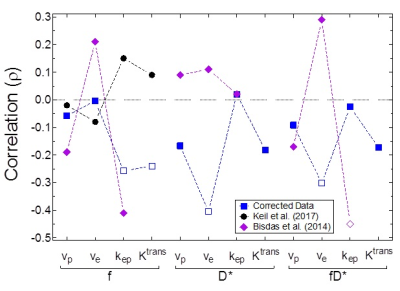
IVIM perfusion parameters, in general, did not correlate with the perfusion parameters obtained from DCE-MRI. Figure 1 shows a weak negative correlation (ρ=-0.058, P=0.573) of the IVIM perfusion fraction (f) to DCE-MRI percent plasma volume (vp). Figure 2 similarly shows a weakly negative correlation (ρ=-0.173, P=0.093) for the comparison of two parameters related to flow (fD* and Ktrans). All reported parameter correlations are shown in Fig. 3 for noise-corrected data measured in this study, along with those reported from two others studies4-5. None of the three studies indicate a trend towards positive correlation between any two sets of parameters.Discussion
Comparisons between IVIM and DCE-MRI quantitative parameters have not been well studied, especially within the human brain. The framework for each of these acquisitions is that they both measure perfusion, albeit by different means. The most intuitive comparison is between blood volume measurements (f versus vp) where the expected positive correlation was clearly not observed (Fig. 1); Figure 3 indicates that none of the three studies report a positive correlation. No discernible pattern appears to emerge from these studies for nearly all parameter comparisons. Bisdas et al. obtained IVIM parameters from a modified bi-exponential decay (perfusion term included D+D*) using a different fit method5. This indicates that regardless of the fitting technique chosen to obtain IVIM parameters, no significant correlation exists.Conclusion
The results presented in this study show that perfusion parameters obtained with IVIM and DCE-MRI are poorly correlated in glioblastomas. This suggests that they are in fact describing different tissue properties and can perhaps be considered complimentary parameters rather than redundant ones. The results further suggest that it may be beneficial to obtain both sets of parameters, rather than one or the other, since they appear to characterize different tissue properties - possibly different aspects of the perfusion process.Acknowledgements
The authors would like to thank Christian Federau for his guidance in establishing our IVIM protocol and data processing methods.
References
1. Padhani AR and Husband JE. Dynamic Contrast-enhanced MRI Studies in Oncology with an Emphasis on Quantification, Validation and Human Studies. Clin Radiol. 2001;56(8):607-620.
2. Le Bihan D, Breton E, Lallemand D, et al. Separation of Diffusion and Perfusion in Intravoxel Incoherent Motion MR Imaging. Radiology. 1988;168(2):497-505.
3. Sigmund EE and Jensen J. (2011). Basic physical principles of body diffusion-weighted MRI. In B. Taouli (Ed.), Extra-Cranial Applications of Diffusion-Weighted MRI (pp. 1-17). New York, NY: Cambridge University Press.
4. Keil VC, Mädler B, Gielen GH, et al. Intravoxel Incoherent Motion MRI in the Brain: Impact of the Fitting Model on Perfusion Fraction and Lesion Differentiability. J Magn Reson Imaging. 2017;46(4):1187-1199.
5. Bisdas S, Braun C, Skardelly M, et al. Correlative assessment of tumor microcirculation using contrast-enhanced perfusion MRI and intravoxel incoherent motion diffusion-weighted MRI: is there a link between them? NMR Biomed. 2014;27(10):1184-1191.
6. Lee EY, Hui ES, Chan KK, et al. Relationship between intravoxel incoherent motion diffusion-weighted MRI and dynamic contrast-enhanced MRI in tissue perfusion of cervical cancers. J Magn Reson Imaging. 2015;42(2):454-459.
Figures