1573
Pipeline for post-processing peripheral nerve DTITina Jeon1, Jerome J Maller2, Maggie M.K. Fung3, and Darryl B Sneag1
1Radiology and Imaging, Hospital for Special Surgery, New York, NY, United States, 2General Electric Healthcare, Melbourne, Australia, 3General Electric Healthcare, New York, NY, United States
Synopsis
The purpose of the study is to evaluate and formalize a post-processing pipeline for DTI of the peripheral nerves using existing open source software suites. Our method integrates image registration, nerve segmentation, and DTI fiber tracking using the FMRIB software library (FSL) and MRtrix3, two popular software suites primarily used in the brain. 6 normal volunteers/patients and 9 nerves were analyzed and image quality was assessed. Using this protocol, image quality significantly improved in addition reducing processing time to 10 minutes using a semi-automated method.
Purpose
Peripheral nerve magnetic resonance diffusion tensor imaging (DTI) is an emerging technique to understand microstructural integrity of both healthy and injured extremity nerves [1-3]. DTI data post-processing is a critical component to reliably interpret acquired DTI data. Although DTI post-processing schemes have been extensively developed for the brain [4-6], peripheral nerve DTI involves its own unique challenges related to off-isocenter distortion, venous contamination and greater propensity for motion of the imaged extremity [7]. The objective of this study was to develop a semi-automated post-processing protocol dedicated to peripheral nerve DTI that involves image registration, segmentation, and streamline tractography.Methods
Subjects and data acquisition: 6 consented healthy volunteers (n=4) and patients (n=2) (age range 24-66 yrs; mean age = 38±18; 1M, 5F) were scanned at the level of the elbow, wrist, or knee on a 3T 60cm bore MR scanner (Discovery MR 750, GE Healthcare, Milwaukee, USA) with a 16-channel flexible extremity array with the patient in the prone, superman position (elbow and wrist) or supine (knee). Imaging parameters: Axial proton density (PD): FOV=12(SI)x12(AP)cm, matrix=144x144, in-plane resolution=0.47x0.47mm2, slice thickness (ST)=0.8mm (no gap), number of slices=40-60, bandwidth=488.3kHz, TR=~4500-5000ms, TE=26-32ms, number of excitations (NEX) =2, imaging time=3-5 min. Axial DTI: Single shot EPI with parallel imaging (factor=2), multiband factor=2, FOV=12(SI)x12(AP)cm, matrix=80x80, in-plane resolution=0.47x0.47 mm2, ST=1.6mm (no gap), bandwidth=1304.7kHz, TR=3400-4500 ms, TE=28-60ms, NEX=3, b-values=0, 600, 1000 sec/mm2, 30 interleaved gradient directions (15 directions each for 600 and 1000 sec/mm2), imaging time=4-6 minutes. Post-processing pipeline for peripheral nerve DTI is summarized in Fig. 1 and was as follows: 1) Image registration of the PD images to non-diffusion weighted images (b0) employing affine linear transformation with FLIRT (FMRIB’s Linear Image Registration Tool) in FSL (fsl.fmrib.ox.ac.uk). 2) The registered PD was then segmented with FAST (FMRIB’s Automated Segmentation Tool) in FSL, outputting 3 classes of tissue types based on image contrast, roughly estimated as nerve/muscle, bone, and fat/water. Non-nerve issue was manually subtracted from the final segmentation. 3) Deterministic tractography was performed using the segmented nerve as a seed producing 10,000 streamlines with MRtrix3 (mrtrix.org). The resulting fibers were masked using the bone and fat/water segmentation from FAST segmentation as an exclusion mask and re-run. Image quality was assessed by: 1) Registration of the PD to the DTI image: The x,y coordinates (same z coordinate) of the median, ulnar and/or common peroneal nerves were located on the B0 image and PD sequence before and after image registration with FLIRT. The coordinates were then subtracted using the distance formula. Distance = √((x2-y2)+(y2-y1)2), where x and y are the coordinates at the center of the nerve. 2) Dice ratios were calculated by subtracting the FAST segmentation from the manually drawn region of interest on the PD image and calculating the percentage overlap of the two images. 3) Tractography: The z-length distance in mm for the streamlines belonging to a particular nerve.Results
Total time to process each data set through the algorithm was approximately 10 minutes. The registration of the nerve markedly improved after image registration with FLIRT (Table 1). 9/9 data sets had a reduction in distance from the x, y coordinates of the corresponding location on the b0 image after registration compared to before (Table 2). Nerve tract length ranged from 12.4-42 mm depending on location of the nerve. Two nerves could not be traced using streamline tractography due to low SNR. Streamline tracking was able to capture a mass-like enlargement of the median nerve (Fig. 2) in the wrist proximal to the carpal tunnel, compatible with a fibrolipomatous hamartoma. Dice ratios (Table 3) of the PD segmentation ranged from 0.64-0.94, with lower overlap ratios in larger diameter nerves.Discussion and Conclusion
In this investigation, we demonstrated a robust semi-automated post-processing protocol for peripheral nerve DTI using existing open source software suites within a clinical time frame. The use of retrospective correction methods is not well established for peripheral nerve DTI. To our knowledge, there has been only one paper comparing the performance of post-processing methods specifically for peripheral nerve using vendor provided post-processing platforms, however, we are the first to assess image quality using open-source software suites which can be used across vendors and allow the user more flexibility. Further improvements in post-processing, including automation and user-friendliness for technologists and radiologists will enable DTI to play a more mainstream role in clinical care.Acknowledgements
Hospital for Special Surgery has an institutional research agreement with GE Healthcare.References
[1] Naraghi et al, Semin Musculoskelet Radiol, 2015; 19:191-200. [2] Guggenberger et al, Radiology 2012, 265: 194-203. [3] Simon et al, J Mag Reson Imaging 2016; 43: 962-969. [4] Jenkinson et al, Neuroimage 2012: 62:782-790. [5] Smith et al, Neuroimage 2006; 31:1487-1505. [6] Soares et al, Front Neuroscience 2013; 7:1-14. [7] Jeon et al, J Mag Reson Imaging 2017.Figures
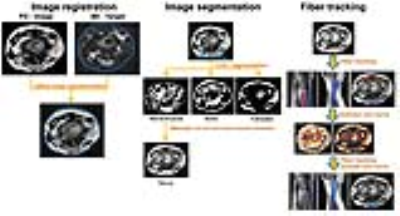
The
post-processing pipeline for peripheral nerve DTI.
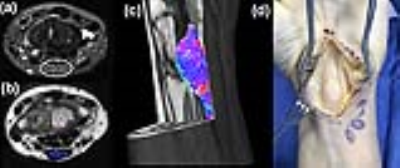
Axial
T2-weighted fat suppressed (a) and axial proton density (b) images demonstrates
mass-like, intrinsic enlargement of the median nerve (white circle) immediately
proximal to the carpel tunnel, with imaging features compatible with a
fibrolipomatous hamartoma. Oblique sagittal tractography image (c) of the
enlarged median nerve overlaid on top of the axial proton density weighted
image at the level of the carpal tunnel is confirmed operatively (d).
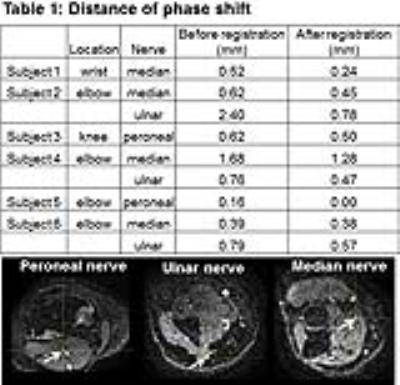
The distance of the
phase shift in the nerve on the PD before registration and after linear affine
registration. The white arrow points to the locations of the peroneal (left),
ulnar (middle), and median (right) nerve that were measured.
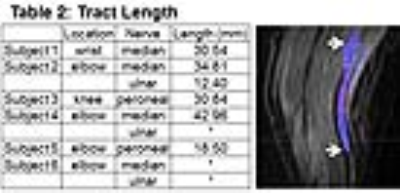
Tract length from
streamline tractography. The white arrows are pointing the z-length of the
streamlines for the ulnar nerve.
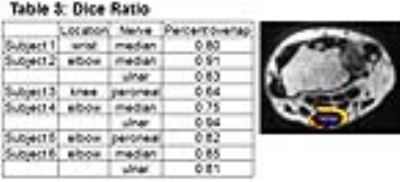
Dice ratios of the
segmented nerve. The yellow is the non-overlapping portion of the nerve overlaid
on the PD image. The purple is the nerve/tumor that overlaps with the manual
segmentation. The dice ratio is calculated by taking the percentage of
overlapping nerve with manual segmentation.