1553
An integrated, semi-automated 3D printed Breast DCE-MRI phantom solution to generate diverse pharmacokinetic curves1Medical Imaging Research Centre, Dayananda Sagar Institution, Bangalore, India, 2Wipro GE healthcare, Bangalore, India, 3Department of Radiology, Columbia University Medical Centre, New York, NY, United States
Synopsis
In vitro phantoms play a critical role in the assessment of novel Dynamic Contrast Enhanced MRI (DCE-MRI) methods related to acquisition and reconstruction, among other advantages such as repeatability and reproducibility. In this work, we demonstrate a 3D printed breast DCE-MRI phantom that is capable of producing diverse kinetic curves as those seen in human patients. The wash-in and wash-out characteristics were controlled through user controlled Ktrans values and the geometry of the phantom respectively. The phantom demonstrated in this work is 3D printed, cost effective, user interface controlled, and integrated with a peristaltic pump to obtain different kinetic curves.
Purpose
The current work aims to develop an integrated DCE-MRI phantom solution that can provide PharmacoKinetic (PK) curves based on user-entered values and the geometry of the phantom. In addition, this solution would involve reproducing these values through PK modelling of the obtained MRI data.Methods and Materials
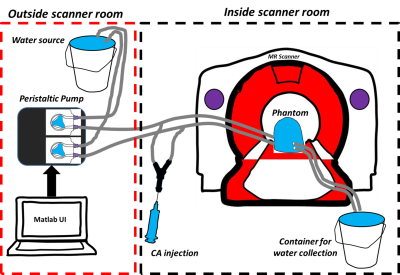
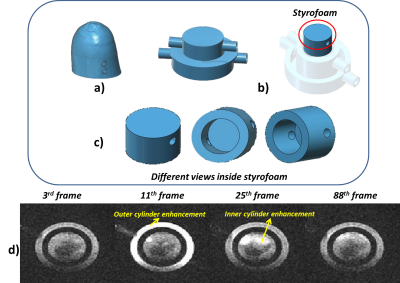
The experimental setup of the 3D printed breast DCE-MRI is diagrammed in Fig. 1. The phantom was connected to the peristaltic pump and controlled through user-entered Ktrans values in a MATLAB (The Mathworks Inc., MA) designed Graphical User Interface (GUI) for controlling both cylinders independently. Ktrans values between 0.3 min-1 to 1 min-1 were varied for generating different PK curves. Combination of these Ktrans values were employed for both inner and outer cylinders. These values were converted into flow rate (corresponding to 15ml/min to 75ml/min for the above Ktrans range) to be pumped from peristaltic pump. Copper Sulphate (CuSo4) was utilized as the contrast agent during the experiments. A detailed description of the breast DCE-MRI phantom design and printing using 3D printer has been previously reported1. Compared to the previous work design shown in1, the inner cylinder was modified to reproduce the poorly perfused region depicted in Fig. 2b. To this end, a piece of styrofoam was placed within the inner cylinder to allow for slow accumulation of the contrast agent during the washout. MRI protocol: Experiments were carried out on GE 1.5T scanner. Relaxivity of the CuSo4 contrast agent was determined prior to performing the DCE-MRI acquisitions. A 3D Spoiled Gradient Recalled Echo sequence with six flip angles and TR/TE=7.6/2.9ms was utilized to obtain variable flip angle images to compute the T10 map. Three dimensional dynamic acquisitions were performed with the DISCO sequence with parameters of TR/TE=6.35/2.98ms, FA=7, number of frames= 47 to 100 with a temporal resolution of 5 sec. Image Analysis: The resulting MR images were processed along with T10 map to determine the corresponding concentration maps. The two compartmental Tofts model was employed for PK modelling. The Arterial Input Function (AIF) required for modelling was generated using the population based AIF equation reported in ref3. Values of volume fraction that were greater than 1 were not included.Results
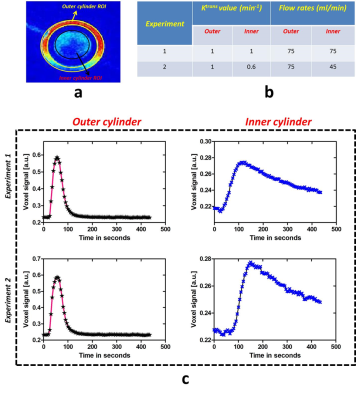
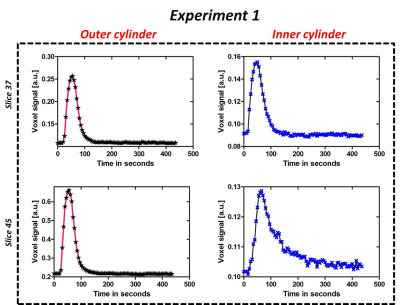
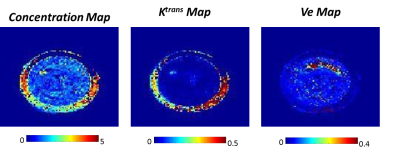
Dynamic MR images of 3D printed breast phantom acquired during and post contrast agent injection (for a particular flow rate) are depicted in Fig. 2d. Each data set was normalized to have a signal intensity range of 0 to 1. It can be observed that outer cylinder shows a greater enhancement as compared to the inner cylinder. Fig. 3b tabulates the two experiments that have been chosen to demonstrate the utility of the phantom. Signal intensity curves obtained from both outer and inner cylinders for the 50th slice selected for different Ktrans values using different flow rates are depicted in Fig. 3c. It can be noticed that the curves with identical flows rates are significantly similar. The curves for the inner ring appear similar for two flow rates but have a significant difference in the time to peak signal intensity, with the slower flow rate having a delayed enhancement. Fig. 4 depicts the voxel signal plots obtained for different slices for a representative experiment 1 (tabulated in Fig. 3b). It can be seen that the curve pattern varies for the inner ring based on the slice position. This variation validates the usage of the Styrofoam insert that provided a pocket for the contrast to accumulate in the inner ring, with a reduced in-flow. This can be visualized through comparing the resulting curves in Fig. 3c and 4. The outer ring does not follow this behaviour due to unrestricted contrast flow. Fig. 5 depicts the concentration and PK maps obtained using Tofts model for experiment 1. It can be noticed that the outer ring shows higher Ktrans values compared to inner ring, as expected.Discussion and conclusion
A semi-automated 3D breast DCE-MRI phantom solution for obtaining diverse dynamic curves has been demonstrated. We have shown different curves that could be obtained for different selection of slices, Ktrans values/flow rates. The phantom behaviour would be impacted by the inter-user variability in the case of a manual injection of contrast agent, synchronization between the injection and acquisition (if not performed as part of a synchronized protocol). These limitations could be overcome by using automated power injector. In conclusion, the current work develops and implements an integrated, cost effective, DCE-MRI phantom solution, including a user interface, 3D printer and peristaltic pump for producing diverse kinetic curves similar to those in patients with reproducibility.Acknowledgements
Department of Science and technology (DST), Govt of India,DST/TSG/NTS/2013/100References
[1]Nithin N Vajuvalli el.al 3D printed Breast DCE-MRI phantom to mimic structure and pharmacokinetics, ISMRM 2017
[2] Manojkumar Saranathan el.al DIfferential subsampling with cartesian ordering (DISCO): A high spatio-temporal resolution dixon imaging sequence for multiphasic contrast enhanced abdominal imaging, JMRI 2012
[3] Parker el.al Experimentally-derived functional form for a population-averaged high-temporal-resolution arterial input function for dynamic contrast-enhanced MRI, MRM 2006
Figures