1551
Integrating Magnetic Resonance Imaging with Live Lung Intravital Microscopy: A Novel Platform to Evaluate the Effect of Radiation on Lung TumorsShampa Chatterjee1, Luis Loza2, Mehrdad Pourfathi2, Sarmad Siddiqui2, Jian Tao1, Harrilla Profka2, Ian Duncan2, Hooman Hamedani2, Kai Ruppert2, Diane Lim3, Yan Liu3, Jose Conejo-Garcia4, Mary Spencer2, Tahmina Achekzai2, Stephen Kadlecek2, and Rahim R. Rizi2
1Physiology, University of Pennsylvania, Philadelphia, PA, United States, 2Radiology, University of Pennsylvania, Philadelphia, PA, United States, 3Sleep Medicine, University of Pennsylvania, Philadelphia, PA, United States, 4Moffitt Cancer Center, Tampa, FL, United States
Synopsis
We propose that, when used in combination with MRI imaging, live lung intravital fluorescence microscopy can be a powerful tool for detecting the effects of radiotherapy on lung tumors. In this study, we monitored pulmonary nodules pre- and post-radiation in a novel murine model (Kras(G12D)/p53fl/fl/myr-p110) with tumor regulation by Cre-recombinase. Using the reporter gene EGFP fluorescence, a significant loss of the tumor was observed post-radiation, which correlated with reduced fluorescent signal from the same region of the lung.
Introduction
Magnetic Resonance Imaging is a powerful technique which has been successfully employed to detect pulmonary tumors. However, the spatial resolution MRI provides is not sufficient to detect a small tumor or clusters of tumor cells, and is incapable of unambiguously discriminating between tumors and other pulmonary lesions. For such high resolution and specificity, molecular imaging by fluorescence microscopy has been employed to visualize lung tumor cells. We therefore hypothesized that combining MRI with live lung fluorescence imaging techniques would allow us to investigate correlations between observations from noninvasive longitudinal imaging and cellular and histologic data. We used a novel murine lung tumor model in which tumor cells express green fluorescent protein. The post-radiation reduction in tumor size correlated with reduced fluorescent signal from the same region of the lung. Such a multi-modality approach allows one to evaluate the effects of radiotherapy not just in terms of tumor size, but in terms of radiation-induced changes in tumor re-organization, metastasis and ultrastructure of the tumor bed.Materials and Methods
Tumors were induced in Kras (G12D)/p53fl/fl/myr-p110 mice of C57BL6 background via the injection of Cre-recombinase virus, as previously described (1). Mice were irradiated at 15 Gy and imaged 14 days later using a 9.4T vertical-bore micro-imaging MRI system (Bruker Inc.). T2-weighted proton images were acquired using a respiratory-gated multi-slice dual-echo RARE pulse sequence (TR/TE1/TE2 = 570/2.1/10.1ms, ETL = 4, NA = 8, matrix size = 192x192, FOV = 30x30 mm2, Bandwidth = 500kHz, 20 slices, 0.8mm slice thickness). Intravital lung imaging was carried out as previously reported (2,3). Mice were anesthetized, the trachea was cannulated, and lungs were ventilated with 5% CO2 in air. The chest was opened, the pulmonary artery cannulated, and the lungs were cleared of blood by gravity-driven flow of Krebs-Ringer bicarbonate solution, KRB (in mM: NaCl 118, KCl 4.7, MgSO4•7H2O 1.2, KH2PO4 1.2, NaHCO3 24.9) supplemented with 10mM glucose and 5% w/vol dextran. Lungs were then dissected free and kept perfused with a peristaltic pump for approx. 10-15 min. Finally, lungs were positioned on the stage of a confocal microscope (BioRad Radiance 2000 laser scanning) and imaged at an excitation wavelength of 488 nm for GFP fluorescence.Results and Discussion
Figure 1 shows serial 1H MRI images of two tumor foci before irradiation, as well as at 2 and 3 weeks post-radiation. Notably, the post-irradiation tumor appears visibly larger and lacks a well-defined boundary; without further study, it is impossible to know if indicates further growth, radiation pneumonitis, fibrotic remodeling, or other consolidation of lung tissue near the tumor. At 3 weeks post-irradiation, the consolidated region appears smaller, suggesting that acute post-irradiation effects have somewhat resolved but leaving significant uncertainty with respect to remaining tumor viability. Pre-radiation live lung imaging of the left lung confirms the presence of tumor nodules as visualized by a green fluorescent signal (Figure 2, bottom left). These nodules are significantly reduced or eliminated post-radiation, suggesting that irradiation has rendered the original tumor non-viable and clarifying the ambiguity which remained after MR imaging alone. However, a lack of sensitivity to the remaining tumor tissue mass and surrounding consolidation makes it impossible to assess changes to lung structure/function relevant to subject morbidity and mortality using this modality alone. Combined (and, ideally, spatially fused) images acquired via both modalities are required to understand the acute and chronic effects of radiation in treating lung tumors. Notably, although the initial tumor fluorescence is largely eliminated, punctate fluorescent structures are observed throughout the upper lobe of the left lung (Figure 3), seeming to indicate the presence of tiny clusters of 10-20 tumors cells. These structures are completely invisible on MRI at this early stage, but suggest a very useful model of recurrent cancer post-radiation. Further work will be required to assess the utility of MRI in following any growth of these post-radiation foci.Conclusion
Live lung imaging allows us to visualize the cellular changes in tumor growth and organization (including metastasis) after radiotherapy. In combination with MRI, this is a powerful tool for correlating data derived from non-invasive longitudinal imaging technologies with that derived from microscopic techniques in order to evaluate tumor fate at the cellular and molecular level.Acknowledgements
References
1. Sheen MR et al, Open Life Sciences 10:85
2. Chatterjee et al. 2006, 13:633-44
3. Noel et al. Am J Physiol Lung Cell Mol Physiol. 2013, 305, L805-18
Figures
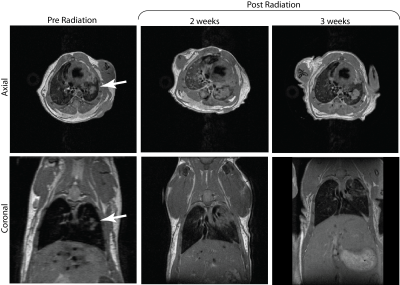
Figure 1. Axial and coronal T2-weighted proton images of
mouse induced with lung tumor in the left lung (white arrow) obtained before
radiation (left), 2 and 3 weeks after radiation. 2 weeks after radiation we see
a growth in the tumor size. However, the tumor reduces in size 3 weeks post
radiation.
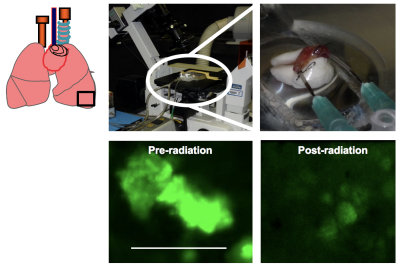
Figure 2.
Green fluorescent signal enables tumor visualization by Intravital Lung
Imaging. GFP indicates that Cre-recombinase excision has been effective, hence
reporter gene GFP is activated.
Upper
panel: Isolated,
ventilated and perfused lungs on the stage of a microscope, with enlarged
version on inset.
Lower
panel: Lungs
from KrasPositive tumor mice were removed and imaged. Tumor nodules can be
visualized. In separate experiments, lungs from KrasPositive tumor mice
subjected to 15 Gy radiation were removed and assessed for tumor size. Low GFP
fluorescence indicates reduced tumor burden; however, sporadic clumps of cells
were observed. Scale bar is 1 mm.