1549
Effect of corrections for image distortion and gradient nonlinearity on longitudinal DTI tumor measurements in breast patients receiving neoadjuvant chemotherapy1University of California San Francisco, San Francisco, CA, United States, 2GE Global Research, Niskayuna, NY, United States
Synopsis
Diffusion weighted imaging has shown promise for assessing tumor response to treatment, but suffers from gradient nonlinearity and image distortion that may adversely affect quantitative accuracy. This work evaluates corrections for image distortion (susceptibility-induced and eddy current) and bias from gradient non-linearity (GN) on breast tumor DTI metrics prior to treatment (T0) and at an early-treatment time point (T1), in six breast cancer patients undergoing neaoadjuvnt chemotherapy. Both GN and distortion correction had significant effects on tumor ADC and FA values at T0 and T1. The addition of distortion correction also improved the alignment of DTI and DCE-MRI tumor ROIs.
Introduction
In studies of breast cancer, DWI increased diagnostic accuracy and showed promise as a biomarker of early treatment response1. However, one limitation of breast DWI is that standard echo-planar imaging (EPI) suffers from geometric distortions resulting from the susceptibility-induced variation in the B0 field and eddy-currents from diffusion-encoding gradients. These distortion effects occur in addition to gradient non-linearity (GN) effects, which have been shown to affect the quantitative accuracy of breast DWI2. A robust method for correcting geometric distortions in the brain utilizes an extra reverse phase gradient (RPG) acquisition to estimate the B0 map3. This correction strategy has also been recently applied to breast DWI4and has was shown to improve the accuracy of diffusion tensor imaging (DTI) metrics measured in a phantom with a similar trend seen for in vivo DTI measurements in breast tumors. However, there is little information about the effects of RPG based correction algorithms on the quantification of breast tumor DTI metrics in longitudinal studies of neoajuvant (pre-surgery) treatment response. This work evaluates the effects of GN correction and the addition of correction for susceptibility-induced distortion and distortion due to eddy currents on diffusion tensor imaging (DTI) metrics measured in malignant breast tumors prior to treatment (T0) and at an early treatment time-point (T1).Methods
In vivo diffusion tensor imaging (DTI) was acquired in 6 patients with locally-advanced breast cancer enrolled in an institutional review board-approved, HIPAA compliant, clinical trial. All patients gave informed consent and DTI data were acquired prior to initiation of treatment and after one cycle of taxane-based therapy. Bilateral axial MRI were acquired on a 1.5T whole body scanner (GE Healthcare, Waukesha, WI) using an 8-channel breast coil (Invivo, Gainesville, FL). A standard echo-planar DTI acquisition was performed (6 directions, b=0,600s/mm2) along with a corresponding reversed-polarity-gradient (RPG) acquisition (1 direction, b=0, 600s/mm2). Standard and corrected parametric ADC and FA maps were calculated from DTI data. The EPI effects (phase-encoding distortion from inherent B0 susceptibility) were corrected using the RPG algorithm3. Eddy-current effects (phase-encoding distortion) were corrected using image registration6. For patient scans, one ROI was defined on the uncorrected DTI slice estimated to contain the largest tumor area. These ROIs were then mapped to the corresponding slice on the corrected ADC and FA maps; minor adjustments were made if needed. DTI ROIs were also mapped to the corresponding slice on DCE-MRI, and the alignment between the ROI and enhancing voxels was assessed using the Dice correlation and the number. Differences between uncorrected and corrected ADC and FA were evaluated using a paired t-test, P<0.05 considered significant. The alignment between uncorrected and corrected (DTI) tumor ROIs and tumor ROIs defined on enhancing regions of DCE-MRI by calculating the both the Dice coefficient and the change in the fraction of DCE-MRI tumor voxels encompassed by the DTI tumor ROIs.Results
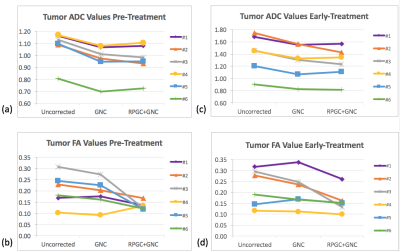
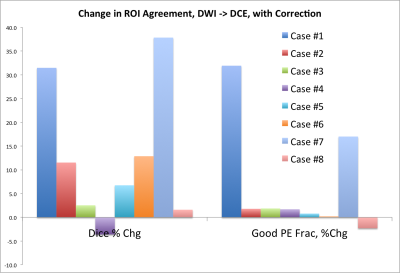
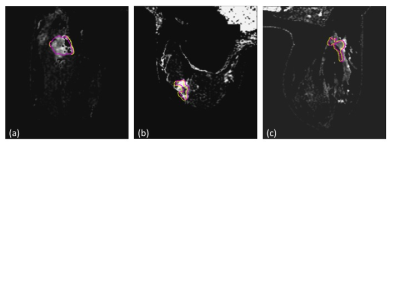
Figure 1. shows the effects of GNC only and GNC+RPG+ EC corrections on mean tumor ADC and FA measurements from the patients at the pre- and early-treatment time points. GNC correction reduced tumor ADC with a significant difference (p<0.001) between pre- and post correction values for both T0 and T1, Addition of RPG and EC corrections to GN correction did not significantly alter tumor ADC, but did significantly decreased FA at T0 (p=0.02). This difference was not significant at T1 (p=0.06). Addition of RPG and EC corrections also improved the alignment of DTI and DCE-tumor ROIs as demonstrated by the increase in Dice coefficient and percent of common voxels between DTI and DCE-MRI tumor ROIs shown in Figure 2. Representative examples of the effects of image distortion correction on the concordance between DTI and DCE-MRI tumor ROIs in different patients are shown in Figure 3.Discussion
Previous work has shown that gradient non-linearity can result in variation of tumor ADC and FA values, and that these variations can be corrected. The changes in tumor FA and ADC after GNC correction measured in this work were consistent with previous findings 4,5. The results of this work show that RPG and EC correction, when applied in addition to GNC have significant effect on tumor FA that paralleled to results previously reported in a phantom with known FA value and can also improve DTI tumor registration with DCE-MRI tumor ROIs.Conclusion
Addition of RPG and EC distortion correction demonstrated measurable changes in ADC and FA values compared to uncorrected values at both T0 and T1. Work is ongoing to evaluate these effects in a larger patient cohortAcknowledgements
The authors acknowledge useful discussion with Ileana Hancu and Jonahtan Sperl, National Institutes of Health Grant U01CA151235 R01Ca190299 and Susan G Komen Grant SAC110017.References
1. Pickles MD, Magn Reson Imaging, 2006 Sep;24(7):843-7.
2. Newitt DC et al, JMRI 2015; 42(4)908-19.
3. Holland D et al, Neuroimage 2010;50(1):175-183.
4. Teruel J et al, Magn Reson Med 2015 74:1138–1144.
5. Wilmes LJ, et al, International Society of Magnetic Resonance in Medicine, 2017, 4936.
6. Tan ET et al, JMRI 2013;38(2)448-53.
Figures