1548
Vascular-induced spin dephasing in real vascular networks reveals useful decay characteristics to differentiate glioblastoma from healthy brain tissue1Neuroradiology, Heidelberg University Hospital, Heidelberg, Germany, 2Molecular Mechanisms of Tumor Invasion (V077), German Cancer Research Center (DKFZ), Heidelberg, Germany, 3E010 Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany
Synopsis
The transverse relaxation attributed to spin dephasing, caused by microscopic field inhomogeneities throughout a single imaging voxel, induced by the BOLD-mechanism, is studied using realistic three-dimensional microvascular structures, attained with fluorescence ultramicroscopy from mouse brains, and custom-written simulations to uncover differences between glioblastoma and healthy brain tissue. The signal attenuation is weaker and more heterogeneous in tumor tissue. Relaxation rates scale differently with varying field strengths or blood properties and the relaxation processes exhibit strong deviations from Lorentzian decay. The results are important for the development of signal processing methods for tumor diagnosis without contrast agents.
Introduction
To model blood vessels in living tissue, extensive research has been conducted about the dephasing effects of cylindrical inclusions of distinct susceptibility in a homogeneous background (see 1,2 for details and references therein). Much focus has been put on correlating individual geometric features, such as the vessel radius or blood volume ratio in tissue, with the NMR relaxation process. In this study, we investigate the influence of real cerebral vasculature in the healthy mouse brain and glioblastoma on spin dephasing during FID and SE experiments. The purpose is to delineate the collective influence of the altered vessel structures in the tumor on the transverse relaxation.Methods
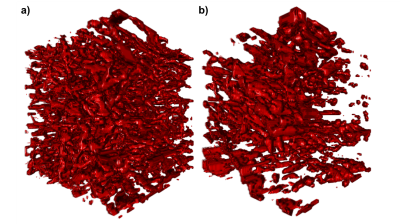
6-8 week old C57Bl/6J mice (n=6) were implanted U87-tumor cells by stereotactic injection. After 21 days, these mice and n=3 additional, healthy mice were injected Lectin-FITC for the fluorescent labeling of vessel lumen with subsequent tissue clearing and selective plane illumination microscopy (SPIM).3 Eighteen representative cubic regions of 0.7mm side length were extracted from each tissue type, respectively, imaged at 3.25µm in-plane resolution and 5µm inter-slice distance (Fig. 1). Using a modified finite perturber method,4 the local magnetic field distortions from vessels, paramagnetic due to deoxyhemoglobin content, are determined in Fourier space using custom-written code in Matlab R2016b. For the central 100µm cube (approximate voxel size in animal MRI), the induced spin dephasing during free induction decay (FID) and spin echo (SE) is simulated with water proton diffusion, implemented through a random walk process in C++11. A wide range of external field strengths and blood properties, such as oxygenation status, are included in the simulated scenarios.Results
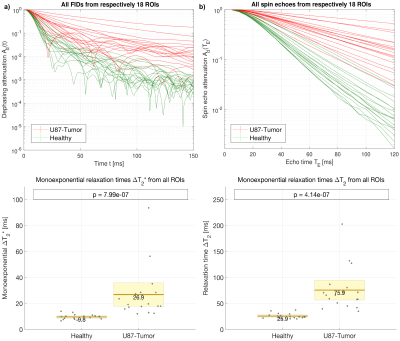
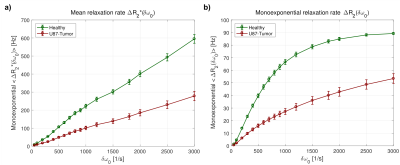
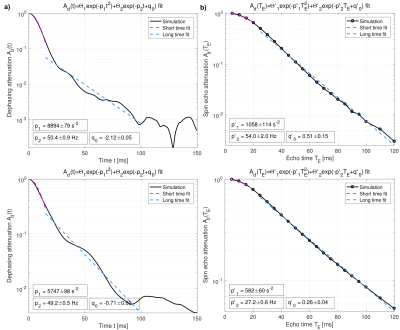
The blood-induced spin dephasing exhibits remarkable differences between tissue types, in the FID as well as in the SE. In healthy tissue, spin dephasing causes stronger relaxation, and simple monoexponential decay modelling reveals higher heterogeneity in the associated changes in relaxation times T2 and T2* in tumor tissue (Fig. 2). The mean relaxation rate changes, ∆R2 and ∆R2*, show different behavior with varying blood susceptibility or field strength B0, summarized in the characteristic off-resonance δω0 (Fig. 3). In agreement with previous experimental and theoretical findings,5 the dephasing effects show obvious deviations from monoexponential relaxation. Both FID and SE dephasing are better described by Gaussian decay for short times and Lorentzian decay with oscillating components for long times (Fig. 4). More elaborate fit models, involving different time regimes and exponential types, show further potential for improvement.Discussion
The collective influence of microvasculature on the transverse relaxation during FID and SE measurements differs in a measurable order of magnitude between glioblastoma and healthy brain tissue, even without the use of paramagnetic contrast agents. The results show that experimental alteration of blood susceptibility during MRI measurements, e.g., by CO2-inhalation, may prove useful in diagnostic imaging.Conclusion
The uncovered differences are important for the development of methods to classify individual voxels from MRI measurements, regarding the likelihood of tumor presence. Furthermore, sophisticated fit models on the produced data can assess the applicability of different analytical signal descriptions to brain and tumor vasculature and aid in the development of new empirical models.Acknowledgements
A. Hahn and F.T. Kurz received funding from grant DFG KU 3555/1-1. F.T. Kurz was also supported by the Hoffmann-Klose foundation of Heidelberg University Hospital.References
1. Dickson JD, Ash TWJ, Williams GB, et al. Quantitative phenomenological model of the BOLD contrast mechanism. J Magn Reson 2011;212:17–25.
2. Troprès I, Pannetier N, Grand S, et al. Imaging the Microvessel Caliber and Density: Principles and Applications of Microvascular MRI. Magn Reson Med 2015;73:325-341.
3. Breckwoldt MO, Bode J, Kurz FT, et al. Correlated magnetic resonance imaging and ultramicroscopy (MR-UM) is a tool kit to assess the dynamics of glioma angiogenesis. eLife 2016;5:e11712.
4. Pathak AP, Ward BD, Schmainda KM. A Novel Technique for Modeling Susceptibility-Based Contrast Mechanisms for Arbitrary Microvascular Geometries: The Finite Perturber Method. Neuroimage 2008;40:1130-1143.
5. Sukstanskii AL, Yablonskiy DA. Gaussian approximation in the theory of MR signal formation in the presence of structure-specific magnetic field inhomogeneities. Effects of impermeable susceptibility inclusions. J Magn Reson 2004;167:56-67.
Figures