1542
MRI exploration of the subventricular region of the third ventricle and its association with neurofibromatosis type-1 and white matter integrity in children with optic pathway glioma1Developmental Imaging and Biophysics Section, Developmental Neurosciences, University College London Great Ormond Street Institute of Child Health, London, United Kingdom, 2Radiology Department, Great Ormond Street Children's Hospital, London, United Kingdom, 3Haematology and Oncology Department, Great Ormond Street Children's Hospital, London, United Kingdom
Synopsis
The lateral subventricular zone has been explored in association with high-grade gliomas, both in-vivo and with MRI. The third ventricle subventricular zone (TVZ) has been explored in-vivo, using immunohistochemistry and microarray analysis, with regard to neurofibromatosis type-1-associated low-grade optic pathway gliomas. This remains unexplored with MRI. This study examined diffusion MRI features of the TVZ and its association with NF1-status and peri-tumour white matter integrity. TVZ features correlated with NF1-status, and peri-tumour white matter integrity. These results suggest that the state of the TVZ environment can potentially indicate whether a sporadic tumour might behave like its less disruptive NF1-associated counterpart.
INTRODUCTION
The subventricular zone is a germinal niche composed of neural stem cells. Due to the self-renewing ability of these cells, the subventricular zone of the lateral ventricle has been explored, in-vivo and with clinical MRI, as a role-player in tumorigenesis 1 and outcome behaviour of high-grade gliomas.2 Recently, studies have identified a contributing role of the subventricular zone of the third ventricle (TVZ) in optic pathway gliomas (OPG) of children with neurofibromatosis type-1 (NF1), as a potential OPG cell-of-origin location.3 However, MRI profiles of the TVZ have not yet been analysed for these tumours. The apparent diffusion coefficient (ADC), indicating diffusion magnitude, and the fractional anisotropy (FA), indicating diffusion directionality, are useful in examining tissue characteristics relative to tumour activity.4,5 The aims of this study were to explore ADC in the TVZ (TVZ-ADC) and its association with NF1-status and tumour invasiveness. The objectives were: 1) determine whether TVZ-ADC correlates with NF1-status, 2) characterise the tumour invasive profile by exploring FA gradients in peritumoural healthy-appearing white matter (WM), and 3) determine whether TVZ-ADC correlates with these FA gradients.METHODS
20 paediatric patients with OPG were imaged on a Siemens 3.0 T Prisma scanner (Siemens, Erlangen), with multi-shell diffusion MRI (b=0,1000,2200 s/mm2, 60 directions per shell) in addition to clinical protocols. Diffusion pre-processing was performed using FSL (FMRIB, Oxford). Optic pathway tractography was performed using multi-shell, multi-tissue constrained spherical deconvolution6,7 with MRtrix3 (Brain Research Institute, Melbourne), utilising an existing protocol.4
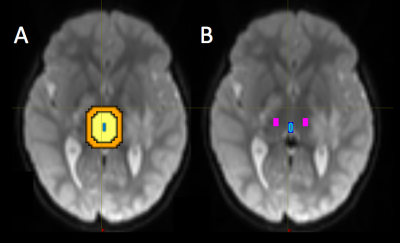
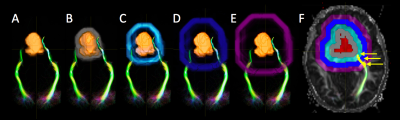
The third ventricle mask was manually drawn in FSL and the TVZ was automatically segmented by dilating the third ventricle mask by 15 mm. A 10 mm restriction was applied inside the dilation to exclude regions potentially affected by partial voluming from cerebral spinal fluid, resulting in a 5 mm dilation. The TVZ ROIs were located within the thalamus and excluded unidentified bright objects and tumour (Figure 1). TVZ-ADCmedian values were recorded. Tumour masks, based on T2-weighted hyperintensity, were manually drawn by a neuro-radiologist using FSL. The tumour mask was dilated by 4 mm increments in all directions, four times, using Matlab, 2016b, (MathWorks Inc., Natick, MA). The tumour dilations that intersected with optic pathway WM were selected and their FAmedian values recorded (Figure 2). The FAmedian gradients, running from the first intersecting WM (4 mm) through to the fourth intersecting WM (total gradient along 16 mm outside the tumour), were calculated after applying a linear fit. TVZ-ADC values were compared according to NF1-status using Students’ t-tests. TVZ-ADC values were then correlated with FAmedian gradient values, while controlling for NF1-status, using partial correlation.
RESULTS
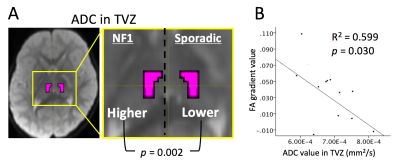
Patient median age was 59 months (range 7 - 172 months). Ten of 20 patients (50%) were NF-1 positive. TZV-ADC of NF1-assoicated cases was significantly higher than in sporadic cases (p = 0.002, two-tailed) (Figure 3A). There a was significant negative correlation between TVZ-ADCmedian and FAmedian gradient, controlling for NF1-status, R2=0.599, p = 0.030 (Figure 3B).DISCUSSION
The higher ADC in the NF1-associated TVZ, and its negative correlation with FA gradients along peritumoural white matter, point to a possible association of TVZ not only with NF1-status, but also with tumour behaviour. NF1-associated OPGs tend to have better prognosis in terms of both visual outcome and tumour progression, whereas sporadic OPGs are more likely to symptomatically progress in comparison.8 The peritumoural biologic and imaging-defined mechanisms involved in tumour behaviour, in the directly adjacent WM, remain relatively unexplored. A shallow FA gradient beyond the T2-defined tumour boundary suggests less locally disrupted white matter. A sharper FA gradient suggests potentially disrupted, and less well-structured, WM adjacent to the tumour. The clinical relevance of these results is highlighted when one considers that, though imaging correlates of symptomatic progression have been previously identified,4 reliable predictive imaging markers of longitudinal symptom change do not yet exist. Furthermore, chemotherapy is generally given to preserve visual function, or in response to a growing tumour. However, response to chemotherapeutic treatment is unpredictable. Determining which child would benefit from, or which child would do just as well without chemotherapy, remains a challenge. These results suggest that NF1-associated OPG cases with lower TVZ-ADC may behave more similarly to sporadic tumours. Conversely, sporadic OPGs with higher TVZ-ADC might behave in a less locally disruptive manner. Incorporating TVZ features into longitudinal MRI analyses of OPG behaviour could add value for non-invasively identifying markers of more disruptive tumour behaviour to predict symptomatic progression.
CONCLUSION
These preliminary findings highlight the potential of examining MRI-defined TVZ characteristics and their involvement in paediatric OPG behaviour. Information of this nature could ultimately assist in improving patient-specific management of these tumours.Acknowledgements
The authors would like to thank the patients who have participated in this study, and the staff at Great Ormond Street Hospital for assistance in acquiring the data. We also thank the University of Minnesota Center for Magnetic Resonance Research for providing the multiband-EPI sequence (http://www.cmrr.umn.edu/multiband). This work was supported by Great Ormond Street Children’s Charity.References
1. Piccirillo SGM, Sottoriva A, Watts C. The role of sub-ventricular zone in gliomagenesis. Aging (Albany NY). 2015;7(10):738-739.
2. Lim DA, Cha S, Mayo MC, et al. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol. 2007;9(4):424-429.
3. Lee DY, Gianino SM, Gutmann DH. Innate Neural Stem Cell Heterogeneity Determines the Patterning of Glioma Formation in Children. Cancer Cell. 2012;22(1):131-138.
4. Hales PW, Smith V, Dhanoa-Hayre D, et al. Delineation of the visual pathway in paediatric optic pathway glioma patients using probabilistic tractography, and correlations with visual acuity. NeuroImage Clin. October 2017 (in press).
5. Nickerson JP, Salmela MB, Koski CJ, Andrews T, Filippi CG. Diffusion tensor imaging of the pediatric optic nerve: Intrinsic and extrinsic pathology compared to normal controls. J Magn Reson Imaging. 2010;32(1):76-81.
6. Tournier JD, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. Neuroimage. 2007;35(4):1459-1472.
7. Mormina E, Arrigo A, Calamuneri A, et al. Optic radiations evaluation in patients affected by high-grade gliomas: a side-by-side constrained spherical deconvolution and diffusion tensor imaging study. Neuroradiology. 2016;58(11):1067-1075.
8. Czyzyk E, Jóźwiak S, Roszkowski M, Schwartz RA. Optic pathway gliomas in children with and without neurofibromatosis 1. J Child Neurol. 2003;18(7):471-478.
Figures