1541
Tumor Metabolism, Diffusion, and Perfusion in Head and Neck Cancer: Pretreatment Multimodality Imaging with DCE-MRI, IVIM DW-MRI, 18F-FMISO PET/CT, and 18F-FDG PET/CT1Medical Physics, Memorial Sloan-Kettering Cancer Center, New York City, NY, United States, 2Radiation Oncology, Memorial Sloan-Kettering Cancer Center, New York City, NY, United States, 3Radiology, Memorial Sloan-Kettering Cancer Center, New York City, NY, United States, 4Medical Physics and Radiology, Memorial Sloan-Kettering Cancer Center, New York City, NY, United States
Synopsis
The aim of this study is to understand the correlation of pretreatment quantitative imaging metrics obtained from multimodality imaging (MMI) techniques, such as DCE-MRI, IVIM DW-MRI, 18F-FMISO PET/CT, and 18F-FDG PET/CT giving us a comprehensive characterization of the tumor in head and neck cancer (HNC) patients. The results show complementary, rather than competitive, information about tumor metabolism, diffusion, and perfusion.
Purpose
Imaging techniques, such as PET/CT and MRI have matured to the extent where it is now possible to non-invasively characterize tumor metabolism, diffusion, and perfusion. Dynamic 18F-fluoromisonidazole (FMISO) PET/CT imaging can reliably assess tumor hypoxia, a major cause of radiation resistance. 18F-fluorodeoxyglucose (FDG) can trace alterations in glucose metabolism associated with cancer cells. Dynamic contrast enhanced (DCE) MRI, on the other hand, assesses the vascular properties of tissue through a gadolinium-based contrast agent (CA). Intravoxel incoherent motion (IVIM) diffusion-weighted MRI (DW-MRI) measures the Brownian water molecular diffusion and capillary perfusion. The aim of this study is to understand the correlation of pretreatment quantitative imaging metrics obtained from multimodality imaging (MMI) techniques, giving us a comprehensive characterization of the tumor in head and neck cancer (HNC) patients.Methods
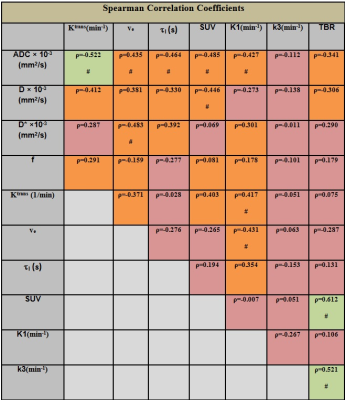
Patients: Our institutional review board approved this retrospective study. Twenty-three HNC patients underwent a total of 92 pretreatment MMI examinations, including IVIM DW-MRI, DCE-MRI, 18F-FDG PET/CT, and 18F-FMISO PET/CT. IVIM DW-MRI data acquisition and analysis: The MRI protocol consisted of multi-planar T1/T2 weighted imaging followed by IVIM DW-MRI on a 3.0T scanner (Ingenia, Philips Healthcare, The Netherlands) using a 20-channel neurovascular phased-array coil. The IVIM DW-MRI images were acquired using a SS-EPI sequence with TR = 4000 ms, TE = 80-100 ms, NEX = 2 and b = 0, 20, 50, 80, 200, 300, 500, 800, 1500, and 2000 s/mm2. The data were fitted using (a) mono-exponential model to calculate the apparent diffusion coefficient (ADC) and (b) bi-exponential model (IVIM DW-MRI) to estimate the diffusion coefficient (D), perfusion fraction (f) and pseudo diffusion coefficient (D*)1, 2. DCE-MRI data acquisition and analysis: A 3D-SPGR-pulse sequence with multiple flip angles of 5°, 15°, and 30° was used for the measurement of precontrast T1. A dynamic series was acquired with TR/TE = 7.0/2.7 ms, phases = 50, flip angle = 15°. An arterial input function was obtained from the carotid artery. The DCE-data were analyzed using a shutter speed model, which estimates the volume transfer constant (Ktrans), interstitial volume fraction (ve), and the mean life time of intracellular water molecules (ti)3. Regions of Interest were delineated on the neck nodal metastases by a team of radiation oncologists and neuroradiologists who were in consensus using ImageJ4. All image processing was performed using in-house MATLAB® software. 18F-FDG PET/CT, FMISO-PET/CT data acquisition and analysis: All patients underwent baseline FDG PET/CT scans, followed by a baseline FMISO dynamic PET/CT5. FDG uptake was calculated as standard uptake value (SUV) corrected by lean body mass. Voxelwise pharmacokinetic modeling of FMISO dynamic PET data was conducted using an irreversible one-plasma two-tissue compartment model to calculate surrogate biomarkers of tumor hypoxia (k3 and Tumor-to-Blood Ratio [TBR]), perfusion (K1) and FMISO distribution volume. All PET segments were spatially co-registered using rigid transformation and tumors segmented using the adaptive threshold algorithm in PET VCAR (Advantage Workstation 4.7; GE Healthcare)6. Statistical analysis: A Spearman correlation analysis was performed between quantitative imaging metrics using standard guidelines7 in which an absolute correlation of < 0.3 was considered weak, 0.3-0.5 moderate, and 0.5-1.0 strong. The significance level was set at p ≤ 0.05.Results
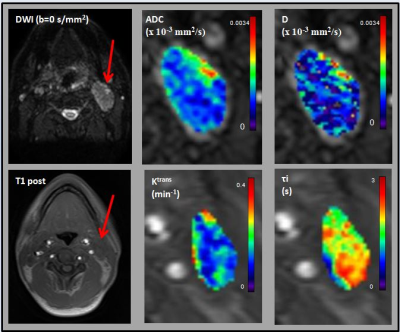
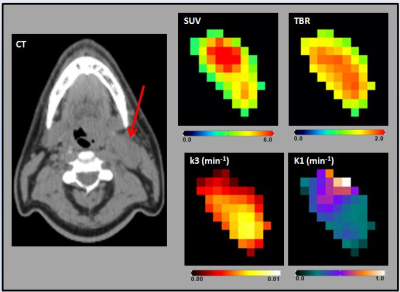
For the 23 patients, the HNC metastatic nodes were analyzed using the 92 pre-treatment MMI datasets. Figure 1 and 2 show the multiparametric maps for ADC, D, Ktrans, tI, SUV, TBR, k3, and K1 from a representative HNC patient. A summary statistics of Spearman correlation parameters are shown in Table 1. There was a significant negative correlation between the mean of the ADC in comparison with K1 and Ktrans (for both, p < 0.05; ρ< -0.4) and a significant positive correlation between K1 and Ktrans (p < 0.05; ρ> 0.4). ADC and D were found to have significant negative correlation with SUV (for both, p < 0.05; ρ< -0.4). A significant negative correlation was found between ADC and tI (p < 0.05; ρ< -0.4). ADC and ve were positively correlated and significant (p < 0.05; ρ> 0.4). K1 and ve were negatively correlated and significant (p < 0.05; ρ<- 0.4). TBR was found significant and positively correlated with SUV and k3 (for both, p < 0.05; ρ> 0.4).Discussion
MMI datasets were used for tumor characterization in HNC. The results show complementary, rather than competitive, information about tumor metabolism; diffusion, and perfusion. Such pretreatment data may have translational applications in treatment planning, prediction of short-term treatment response or outcome, and monitoring treatment.Conclusion
MMI data will further improve our understanding of tumor biology in-vivo and help us unravel new treatment strategies.Acknowledgements
This work was supported by the MSKCC internal IMRAS grant and in part through the NIH/NCI Cancer Center Support Grant: P30 CA008748.References
1. Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401-7. doi: 10.1148/radiology.161.2.3763909. PubMed PMID: 3763909.
2. Lu Y, Jansen JF, Mazaheri Y, Stambuk HE, Koutcher JA, Shukla-Dave A. Extension of the intravoxel incoherent motion model to non-gaussian diffusion in head and neck cancer. Journal of magnetic resonance imaging : JMRI. 2012;36(5):1088-96. doi: 10.1002/jmri.23770. PubMed PMID: 22826198; PubMed Central PMCID: PMC3482143.
3. Kim S 1, Quon H, Loevner LA, Rosen MA, Dougherty L, Kilger AM, Glickson JD, Poptani H. Transcytolemmal water exchange in pharmacokinetic analysis of dynamic contrast-enhanced MRI data in squamous cell carcinoma of the head and neck. J Magn Reson Imaging. 2007 Dec;26(6):1607-17. 4. Rasband WS. ImageJ. Bethesda, Maryland, USA: U. S. National Institutes of Health; 1997-2016.
5. Greco C, Nehmeh SA, Schöder H, Gönen M, Raphael B, Stambuk HE, Humm JL, Larson SM, Lee NY. Evaluation of different methods of 18F-FDG-PET target volume delineation in the radiotherapy of head and neck cancer. Am J Clin Oncol. 2008 Oct;31(5):439-45. doi: 10.1097/COC.0b013e318168ef82.
6. Grkovski M, Lee NY, Schöder H, Carlin SD, Beattie BJ, Riaz N, Leeman JE, O'Donoghue JA, Humm JL. Monitoring early response to chemoradiotherapy with <sup>18</sup>F-FMISO dynamic PET in head and neck cancer. Eur J Nucl Med Mol Imaging. 2017 Sep;44(10):1682-1691. doi: 10.1007/s00259-017-3720-6. Epub 2017 May 24.
7. Cohen J. The statistical power of abnormal-social psychological research: a review. JAbnorm Social Psychol. 1962;65:145-53 [PMID: 13880271]
Figures