1539
Dual-modality molecular imaging of choline kinase expression in lung cancer1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA, United States, 3Surgery, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
MR spectroscopy of tumors show elevated tCho resonances, reflecting increased levels of phosphocholine. This arises from overexpression of choline kinase (ChoK), which can be detected in breast tumor models using targeted near-infrared (NIR) probes and fluorescence optical imaging. This study translates these findings into lung cancer models, measuring elevated ChoK expression and activity in murine and human lung cancer cells and elevated ChoK levels in spontaneous canine adenocarcinomas. Dual modality molecular imaging could be employed using MRI and MRS for tumor staging, followed by NIR imaging for intraoperative surgical guidance, margin detection, and residual tumor removal, increasing patient survival.
INTRODUCTION
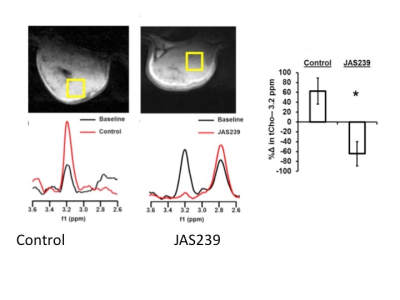
MR spectroscopy has been instrumental in defining lipid metabolic changes in tumor development and progression. These include increases in choline metabolites that can be observed using 31P (phosphocholine and phosphoethanolamine, PC and PE) and 1H MRS (total choline, tCho)1. While MRS remains the best method to assess lipid metabolism noninvasively, difficulties exist in interpreting steady-state metabolite levels due to the overlap of multiple metabolites in the tCho resonance at 3.2 ppm, the upregulation of competing anabolic and catabolic pathways and competition with transport mechanisms2. One approach to this has been to develop radiolabeled choline analogs using 11C and 18F for PET imaging. These have proven to be useful in the assessment of tumors that are not FDG avid, such as prostate cancer3. An additional approach has been to design optical probes for detecting lipid metabolism in vivo using near infrared fluorescence imaging2. We have constructed a series of near-infrared (NIR) choline mimetics that function as inhibitors of choline kinase (ChoKα), the primary enzyme that leads to PC and tCho elevations in tumors. We have demonstrated that the probe JAS239 accumulates in ChoKα-overexpressing breast tumor models and allow delineation of tumor margins in vivo by binding and inhibiting ChoKα4. JAS239 was used diagnostically as an adjunct to MRS to monitor response to chemotherapy, or therapeutically at higher concentrations, where its efficacy was assessed using in vivo MRS to measure decreased tCho (Figure 1). In the present study, we extend this work to lung cancer, demonstrating the overexpression of ChoKα in lung cancer cells and tumors in both small and large animals, with the goal of translating this work to a canine clinical population with spontaneous-occurring lung cancer.METHODS
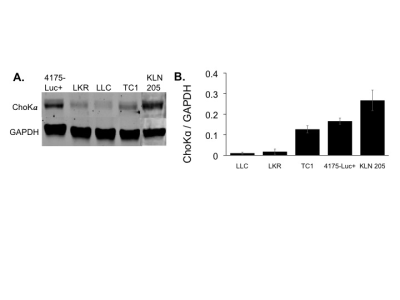
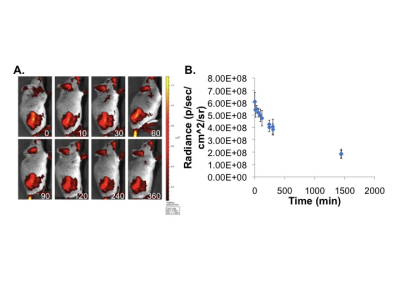
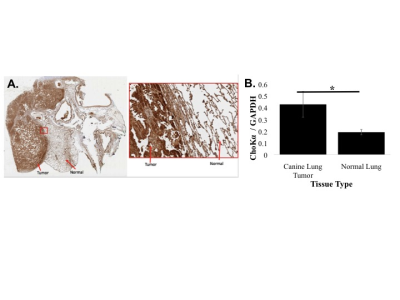
ChoKα expression was measured in human (A549, H1299 and H460) and murine lung cancer cell lines LKR, LLC, TC1, KLN 205) using Western blotting. ChoKα expression was measured in spontaneous canine adenocarcinomas from 3 patient dogs using Western blotting and immunohistochemistry. JAS239 was synthesized as previously described2. Probe toxicity was assessed using the MTT assay. ChoKα inhibition was determined using 14Cho uptake and phosphorylation in whole cells followed by TLC and autoradiography. 1H MRS was performed on a 9.4 T horizontal bore system. Spectra were acquired using a PRESS sequence on a 3x3x3 mm3 voxel placed within the tumor: TR=3000 ms, TE1=12.68 ms, TE2=10.01 ms, number of averages=128, complex points=4096, spectral width=4000 Hz, acquisition time 6 min 24 sec. Water suppression was performed using VAPOR. An unsuppressed spectrum served as a concentration reference.RESULTS
In lung cancer cells, ChoKα expression was comparable to breast cancer, with the KLN 205 murine cell line expressing the highest levels (Figure 2). Using the MTT assay, KLN 205 cells showed an IC50 of 3.125 µM to JAS239, compared to the 10 µM in 4175-Luc+ breast cancer cells. In a 14C-choline assay, JAS239-treated KLN 205 cells had significantly reduced 14C-PC production. In vivo optical imaging of KLN 205 tumors showed JAS239 accumulation and tumor margin delineation (Figure 3). In spontaneous canine lung tumors, immunohistochemistry showed that ChoKα is significantly overexpressed compared to normal canine lung tissue, also confirmed by Western blotting (Figure 4).DISCUSSION
ChoKα is an established biomarker associated with aggressive phenotype, high histological tumor grade, and poor clinical outcome in many human cancers. ChoKα is overexpressed in 60% of human lung tumors5. In murine lung cancer cells, ChoK expression is variable, but higher than positive controls in some tumors. Expression in spontaneous canine tumors is also variable, but fairly homogeneous and consistently significantly higher than normal tissue. Lung cancer is the leading cancer-related cause of death and the third most diagnosed cancer in the U.S. Tumor resection is the most effective approach to cure patients with local disease, but visual inspection and finger palpation is insufficient for detecting tumor margins or micrometastases, leading to local recurrence6. A dual modality imaging approach employing MR spectroscopy with directed biopsy to identify lung tumors with high ChoKa expression, followed by intraoperative imaging using NIR fluorescence with MR guidance could significantly impact on margin detection and patient survivalCONCLUSIONS
ChoKα expression profiling of lung cancer will aid in the translational application of intraoperative molecular imaging for detection and margin delineation of lung tumors in a real-time clinical setting.Acknowledgements
We acknowledge the support of the Small Animal Imaging Facility at the University of Pennsylvania, the Pathology Core Laboratories of The Children’s Hospital of Pennsylvania for histopathology and Veterinary School Pathology services headed by Amy Durham. Financial support is provided by the NIH R01 EB018645 and by a pilot grant from Penn's Institute for Translational Medicine and Therapeutics Translational Bio-Imaging Center (TBIC).References
1. Glunde K, Bhujwalla ZM, Ronen SM. Choline metabolism in malignant transformation. Nat Rev Cancer 2011;11(12):835-48.
2. Arlauckas SP, Popov AV, Delikatny EJ. Direct inhibition of choline kinase by a near-infrared fluorescent carbocyanine. Mol Cancer Ther 2014;13(9):2149-58.
3. Kwee SA, DeGrado TR, Talbot JN, et al. Cancer imaging with fluorine-18-labeled choline derivatives. Semin Nucl Med 2007;37(6):420-8.
4. Arlauckas SP, Kumar M, Popov AV, et al. Near infrared fluorescent imaging of choline kinase alpha expression and inhibition in breast tumors. Oncotarget 2017;8(10):16518-30.
5. Ramirez de Molina A, Rodriguez-Gonzalez A, Gutierrez R, et al. Overexpression of choline kinase is a frequent feature in human tumor-derived cell lines and in lung, prostate, and colorectal human cancers. Biochemical & Biophysical Research Communications 2002;296(3):580-3.
6. Aliperti LA, Predina JD, Vachani A, Singhal S. Local and systemic recurrence is the Achilles heel of cancer surgery. Ann Surg Oncol 2011;18(3):603-7.
Figures