1537
Assessment of treatment response of lymphoma in an animal model with in vivo MR elastography1Radiology, Charité - Universitätsmedizin Berlin, Berlin, Germany, 2Medica Department of Hematology, Oncology, and Tumor Immunology, and Molecular Cancer Research Center (MKFZ), Charité - Universitätsmedizin Berlin, Berlin, Germany, 3Medical Informatics, Charité - Universitätsmedizin Berlin, Berlin, Germany, 4Berlin Institute of Health (BIH), Berlin, Germany, 5Max-Delbrück-Center for Molecular Medicine (MDC), Berlin, Germany
Synopsis
In this feasibility study, we have characterized the mechanical properties of lymphoma directly in the cervical lymph nodes with in vivo multifrequency MRE for the first time. Both MRE and diffusion weighted imaging were used to investigate the tumor's response to chemotherapy. We found that lymphomas stiffened 24 hours after chemotherapy which was accompanied by increased apparent diffusion coefficient (ADC) and reduced tumor volume. Wave speed obtained from MRE is sensitive in detecting the mechanical response of lymphoma to chemotherapy. Observed tumor stiffening post treatment needs to be validated by larger group size and should be explained by histological analysis.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common aggressive Non-Hodgkin’s lymphoma. Despite cure rates of about 50-60%, major challenges in DLBCL remain due to the lack of reliable, biology-anchored prognostic markers, actionable lesions and effective novel treatment strategies. Magnetic resonance elastography (MRE) is a noninvasive imaging technique (1) used to investigate the biomechanical properties of soft tissues and other tumors in vivo. In this study, in vivo MRE was applied to a lymphoma mouse model prior to and post chemotherapy in order to assess the mechanical response of the tumor – as a candidate predictor of outcome and potentially novel target in lymphoma therapy.Methods
10 wild-type C57BL/6 mice were intravenously transplanted with primary Eµ-myc transgenic mouse lymphomas (1x106 cells per mouse). In vivo MRE was conducted at 7 T small animal scanner (Bruker, Biospec, Ettlingen, Germany) using 5 harmonic frequencies (1000 to 1400 Hz, 100 Hz increment). The vibration was generated by a nonmagnetic piezoceramic-actuator and transferred to the cervical lymph nodes via a head holder. The 3D wave field was recorded using a single-shot EPI based sequence implemented with "SLIM" acquisition strategy (2). Total acquisition time for 9 consecutive slices of 0.2×0.2×1 mm3 resolution, 5 frequencies, 8 wave dynamics, 3 signal averagings was 4.5 minutes. MRE wave data were reconstructed using the wave-number-based inversion method as detailed in (3), yielding a parameter map of the shear wave speed (c), which represents mainly the tissue stiffness. We also included diffusion weighted imaging (DWI) in the imaging protocol with b values of 0, 800 and 1.200 s/mm2, 3 orthogonal directions and 2 signal averagings, total acquisition time was 2.5 min, and apparent diffusion coefficient (ADC) map was obtained. Additionally, tumor volume was assessed using data obtained from the anatomical T2-rare sequence with 0.078× 0.078 mm in-plane resolution. The whole imaging protocol was applied twice: the first time when the axillary lymph nodes behind or under the shoulder became palpable (approx. after 10-14 days), and a second time 24 hours after Cyclophosphamide (CTX; an alkylating anticancer chemo agent) treatment (300 mg/kg) which was administered subcutaneously. All imaging protocols (MRE, DWI and T2 RARE) contain 9 axial imaging slices with identical slice position and covering the entire cervical lymph nodes.Results
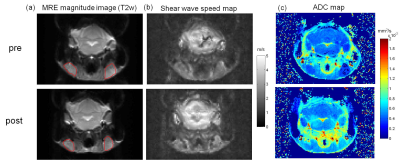
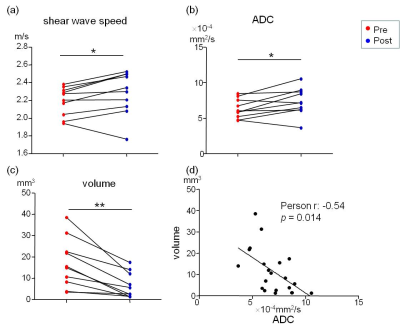
Fig. 1 shows the MRE magnitude image (T2w), shear wave speed map (c-map, c in m/s), and ADC map of a representative mouse pre- and post-CTX treatment. In the c map, the cervical lymph mass are visibly stiffer than the ones prior to treatment. From the ADC maps also follows a noticeable increase in the diffusivity in the lymphoma after chemotherapy. Group mean of wave speed obtained from MRE are significantly lower in the initial tumor state (pre) than post-treatment (pre: 2.19±0.16 m/s vs. post: 2.28±0.24 m/s, P=0.049). Significantly increased values were obtained for ADC after treatment (pre: 6.34 ± 1.34´10-4 mm2/s vs. post: 7.36 ± 1.92´10-4 mm2/s, P=0.04). Additionally, tumor volume, calculated using the standard formula: v=a2b/2, where a=shortest and b=longest diameter, also showed an uniform reduction in all mice in response to chemotherapy (pre: 17.1±11.5 mm3 vs. post: 6.6±5.9 mm3, P = 0.002. We also observed a negative correlation between the ADC and the tumor volume at both pre- and post-treatment time points (Pearson r = -0.54, P=0.014). All imaging data acquired before and after treatment and the correlation analysis are presented in Fig. 2.Discussion
In this study, we have found that the lymphomas stiffened in response to chemotherapy. This results is in contrast to (4) where a reduction in stiffness was reported. We think the discrepancy is probably resulted by differences between the specific lymphoma cell models and the distinct host environments, since ectopic implantation was used in (4). A post-treatment increase in ADC has been reported in the literature (5, 6), and might be explained by alterations in the extracellular/intracellular volume ratio after apoptosis (7). To validate this hypothesis, we will perform immuno-histological analyses for proliferation and apoptosis, and correlate the obtained results with in vivo imaging findings.Conclusion
In this feasibility study, we have characterized the mechanical properties of DLBCL directly in the cervical lymph nodes with in vivo MRE for the first time. Wave speed obtained from MRE is sensitive in detecting the mechanical response of lymphoma to chemotherapy, Observed tumor stiffening post treatment needs to be validated by larger group sizes and should be explained by histological-structural analysis.Acknowledgements
No acknowledgement found.References
1. Muthupillai R, Ehman RL. Magnetic resonance elastography. Nat Med. 1996;2(5):601-3.
2. Klatt D, Johnson CL, Magin RL. Simultaneous, multidirectional acquisition of displacement fields in magnetic resonance elastography of the in vivo human brain. J Magn Reson Imaging. 2015;42(2):297-304.
3. Tzschatzsch H, Guo J, Dittmann F, et al. Tomoelastography by multifrequency wave number recovery from time-harmonic propagating shear waves. Med Image Anal. 2016;30:1-10.
4. Pepin K, Ansell S, Ehman R, McGee K. MR Elastography as a Predictor of Therapeutic Response: Assessment in Non-Hodgkin's Lymphoma (NHL). Med Phys. 2015;42(6):3632-.
5. Huang MQ, Pickup S, Nelson DS, et al. Monitoring response to chemotherapy of non-Hodgkin's lymphoma xenografts by T-2-weighted and diffusion-weighted MRI. Nmr Biomed. 2008;21(10):1021-9. 6. Lee SC, Poptani H, Pickup S, et al. Early detection of radiation therapy response in non-Hodgkin's lymphoma xenografts by in vivo H-1 magnetic resonance spectroscopy and imaging. Nmr Biomed. 2010;23(6):624-32.
7. Hamstra DA, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: a biomarker for treatment response in oncology. J Clin Oncol. 2007;25(26):4104-9.
Figures