1528
Early biomarkers of response to neoadjuvant chemotherapy in lung cancer: preliminary data from a multicenter international study1CRUK Imaging Centre, Institute of Cancer Research, London, United Kingdom, 2MRI Unit, The Royal Marsden NHS Foundation Trust, Sutton, United Kingdom, 3VU University Medical Center, Amsterdam, Netherlands, 4Humanitas University, Milan, Italy, 5The Lung Unit, The Royal Marsden NHS Foundation Trust, Sutton, United Kingdom, 6European Organisation for Research and Treatment of Cancer Headquarters, Brussels, Belgium
Synopsis
Whole tumor ADC histogram parameters were assessed as early response biomarkers to platinum-based neo-adjuvant chemotherapy in 14 patients with non small cell lung cancer. On completion of treatment, 3 of 11 patients with DW-MRI at baseline and day 14 were classed responders by RECIST criteria. At Day 14 of treatment, there was a significant reduction in ADC metrics in responders (2 of 3 beyond limits of agreement) compared to non-responders (2 of 11 beyond limits of agreement). An increase in ADC 75th centile (indicating more voxels with higher ADC values), was consistent with necrosis; non-responders did not show this change.
Background
Lung cancer is the most common cause of cancer death worldwide, with around 1,590,000 deaths from lung cancer in 2012 (19% of the total)1.The ability to recognize response or non-response early would enable a change in treatment plan when other management options are still available. The apparent diffusion coefficient (ADC) derived from diffusion-weighted MRI is proving an invaluable biomarker in a range of tumor types2 especially when whole tumor histogram parameters are used to identify response. To date, derivation of this data in the lung has been difficult because of implementation of an echo-planar based protocol in the chest where respiratory motion and distortion from susceptibility effects from air in lungs considerably degrades the images. We have implemented a free breathing protocol for use in thoracic imaging across a multivendor platform and established the reproducibility of the ADC measurements in a multisite, multivendor setting (CoV 7.1%, median LoA -18.0 to 21.9%, 75th centile -18.7 to 22.5%)3.Aim
To determine the changes in volume of DWI derived lesions and ADC distribution in non small cell lung cancer (NSCLC) treated with platinum-based neoadjuvant chemotherapy at 14 days post-treatment and relate them to response as identified by RECIST at the end of 3 cycles of treatment.Methods
14 patients (9 male, 5 female) aged 53-78 years (mean 65.9 ± 7.1 years) at enrollment in 3 centres (Royal Marsden Hospital (RMH), London; Humanitas, Milan; VUMC Amsterdam) were recruited to this prospective trial (EORTC 1217). 3D T1-W imaging and 2D diffusion-weighted imaging (b= 100, 500, 800 s/mm2) were done to cover the entire chest from lung apices to hemidiaphragms with short tau inversion recovery fat suppression (TI = 180 ms). Tumors were manually segmented with regions of Interest (ROIs) around the tumor on every slice of the computed b800 images where tumor could be identified4. Voxel distributions of ADC were interrogated for 25th, 50th and 75th centile values, IQR, skew and kurtosis at both time points and % change in these parameters was calculated. The DWI-derived lesional volume and their changes were also documented from ROIs on DW-MR images with volumes calculated by multiplying the number of voxels by voxel dimensions. Longest uni-dimensional tumor measurements on CT (n=10 cases at all 3 time points) and MRI (n=4 cases at all 3 time points) at these time-points and immediately post-treatment were used to record response by RECIST criteria. This exploratory evaluation was not specified in the study protocol, and all data were analyzed by investigators at the lead site.Results
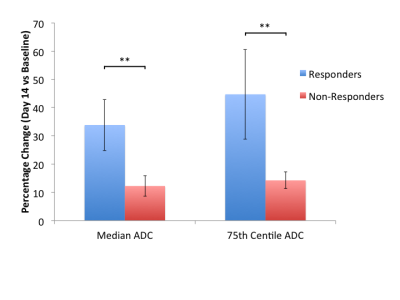
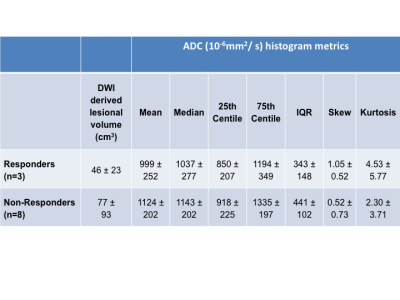
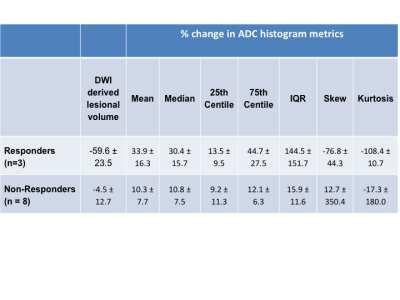
11 of the 14 patients recruited to the study had DWI at baseline and at 14 days post-treatment, with one patient withdrawing from the study prior to imaging and two patients unable to tolerate MRI scans. Baseline DWI derived lesional volumes and ADC parameters are given in Table 1. 3 tumors were considered responders by RECIST criteria and 8 were non-responders. At 14 days, 2 responders and 2 non-responders had a change in median ADC above the limits of agreement set by this consortium while 2 responders and 1 non-responder had a change in 75th centile greater than the limits of agreement (Fig. 1). The percentage change of ADC mean, median, 75th centile and IQR at day 14 versus baseline was significantly higher in responders compared with non-responders (one-tailed t-test unequal variance p = 0.009, 0.004, 0.004 and 0.01 respectively, Table 2).Discussion and Conclusion
NSCLC responded poorly to platinum-based neoadjuvant chemotherapy as assessed by RECIST criteria at the end of chemotherapy. There was a large variation in percentage volume reduction for DWI-derived lesional volume in patients classified as responding by RECIST. In RECIST responders, there was a significant reduction in ADC metrics as early as 14 days after initiation of chemotherapy. The increase in the 75th centile indicated an increase in number of voxels with higher ADC values, consistent with necrosis; the non-responders did not show this change. Early identification of response using ADC histograms may be helpful in decision-making on whether to continue administration of neoadjuvant chemotherapy.Acknowledgements
The research leading to these results has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement number 115151, resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in kind contribution. We also acknowledge CRUK and EPSRC support to the Cancer Imaging Centre at ICR and RMH in association with MRC and Department of Health C1060/A10334, C1060/A16464 and NHS funding to the NIHR Biomedical Research Centre and the Clinical Research Facility in Imaging.References
1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013.
2. Thoeny HC, Ross BD. Predicting and monitoring cancer treatment response with diffusion-weighted MRI. J Magn Reson Imaging. 2010 Jul;32(1):2-16.
3. Weller A, Papoutsaki MV, Waterton JC, Chiti A, Stroobants S, Kuijer J, Blackledge M, Morgan V, deSouza NM. Diffusion-weighted (DW) MRI in lung cancers: ADC test-retest repeatability. Eur Radiol. 2017 Apr 10.
4. Blackledge MD, Leach MO, Collins DJ, Koh DM. Computed diffusion-weighted MR imaging may improve tumor detection. Radiology. 2011 Nov;261(2):273-81.
Figures