1520
Characterization of endometrioid adenocarcinoma microcirculation using distributed parameter model in DCE MRI1West China Second University Hospital, Chengdu, China
Synopsis
Objective: To clarify the features of vascular proliferation and permeability in endometrioid adenocarcinoma. Methods: The DCE-MRI was applied to 55 women who confirmed as endometrioid adenocarcinoma with postoperative pathology. The receiver operating characteristic (ROC) analysis was employed using parameters derived with the DP model to differentiate tumor and normal myometrium and assess the diagnostic efficiency of these parameters. Results: E and PS in tumor was lower. F in tumor was faster. Vp and Ve in tumor were lower. Areas under ROC curve (AUCs) for E and PS attained values of 0.906 and 0.844. AUCs for F attained value of 0.548. Vp and Ve in tumor with AUC values of 0.796 and 0.871. Conclusion: The permeability of vascular wall was significantly lower in endometrioid adenocarcinoma, and the vascularity was moderately lower, suggestive of very different cell growth environment in endometrioid adenocarcinoma in comparison with most solid tumours.
Introduction
To explore the features of vascular proliferation and permeability in endometrioid adenocarcinoma using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and distributed parameter (DP) model.
Methods
This study was approved by the ethical review board and informed consent was obtained from all patients. From December 2016 to August 2017, fifty-five female adults were recruited from the prospective study, who were subjected to diagnostic curettage, were suspected endometrial cancer and underwent preoperative pelvic DCE-MRI examination. All patients were treated with bilateral salpingo-oophorectomy and hysterectomy. Postoperative pathology confirmed that they were endometrioid adenocarcinoma. Regions of interest were drawn by radiologists and the data was analyzed retrospectively using MItalytics (Fitpu Healthcare, Singapore), where DP model was utilized to explore the microcirculation features of endometrioid adenocarcinoma. Blood flow (F), permeability surface area product (PS), plasma space fractional volume (Vp), extravascular extracellular space fractional volume (Ve), and extraction fraction (E) were derived, and receiver operating characteristic (ROC) analysis was employed to differentiate tumor and normal myometrium and assess the diagnostic efficiency of these parameters.Results
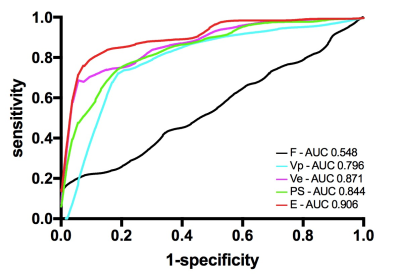
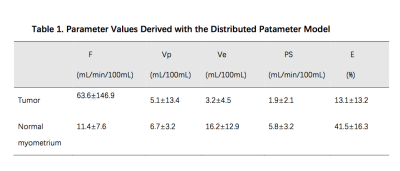
E and PS in tumor was lower than normal myometrium. F in tumor was faster than normal myometrium. Vp and Ve in tumor were lower than normal myometrium (Table 1). Areas under ROC curve (AUCs) for E and PS attained values of 0.906 (95% CI: 0.860, 0.951) and 0.844 (95% CI: 0.784, 0.905) respectively. AUCs for F attained value of 0.548 (95% CI: 0.354, 0.549). Vp and Ve in tumor with AUC values of 0.796 (95% CI: 0.719, 0.872) and 0.871 (95% CI: 0.817, 0.924) respectively(Figure 1). Among all the parameters derived, E attains the largest AUC value, indicating the highest diagnostic efficiency, where the threshold determined by Youden Index was 33.30% with sensitivity 0.805 and specificity 0.925.Discussion
This is, to our knowledge, the first research comparing intra-tumour angiogenesis and myometrium microvessel function based on quantitative DCE-MRI in endometrioid adenocarcinoma patients. Endometrial carcinoma was reported that immunohistochemical marker microvessel density (MVD) was correlated to markers of tumour tissue perfusion and permeability obtained from DCE-MRI kinetic model measurements. However, the relationship between the vascular quantities obtained by immunohistochemical marker and vascular function obtained by DCE-MRI has not yet been clarified. The parameters from quantitative DCE-MRI can provide quantitative information on tissue vascularity, which can help in endometrial cancer detection and tumour grading assessment. As a two-compartment model assuming a distributed vascular cylindrical symmetric compartment, DP were first formulated and solved for their impulse outflow functions for analysis of arterial-venous sampling experiments, and has been shown to be significantly advantageous over other DCE models. Comparing with normal myometrium, Ve in tumor was significantly lower (AUC 0.871), indicating the increased tumor parenchymal cells. F is higher, but AUC is only slightly >0.5. Interestingly, Vp in tumor was moderately lower (AUC 0.796) and permeability in tumor was significantly lower (AUC for E 0.906, for PS 0.844), which is somewhat puzzling, because the tumor vessels are generally considered more vascular and more leaky than normal vessels. This may suggest a very different cell growth mechanism in endometrioid adenocarcinoma, which may be related to the hypoxic environment in endometrioid adenocarcinoma. The hypoxic endothelial cells may cause the reduction in permeability. Tumor hypoxia is described as a characteristic feature of many solid tumours, where dysfunctional blood vessels in the tumor were incapable to restore oxygenation. Nevertheless, most solid tumours are featured by increased vascularity and increased leakiness, to support a higher supply of oxygen and nutrition for tumour cell growth.Conclusion
Comparing with normal myometrium, the permeability of vascular wall was significantly lower in endometrioid adenocarcinoma, and the vascularity was moderately lower, suggestive of very different cell growth environment in endometrioid adenocarcinoma in comparison with most solid tumours.Acknowledgements
This study was supported by The Medicial Key Laboratory of Gynecological Oncology, Sichuan Province, Research Funds at the Department of Radiology, West China Seconnd University Hospital.References
[1] Haldorsen IS, Stefansson I, Gruner R, et al. Increased microvascular proliferation is negatively correlated to tumour blood flow and is associated with unfavourable outcome in endometrial carcinomas. Br J Cancer. 2014;110(1): 107–114.
[2] Ippolito D, Minutolo O, Cadonici A, et al. Endometrial cancer : diagnostic value of quantitative measurements of microvascular changes with DCE-MR imaging. MAGMA. 2014;27(6): 531–538.
[3]Vicini P, Cobelli C. A priori identifiability of distributed models of blood-tissue exchange. Ann Biomed Eng 1999;200–207.
[4]Koh TS, Bisdas S, Koh DM, et al. Fundamentals of Tracer Kinetics for Dynamic Contrast-Enhanced MRI. J Magn Reson Imaging. 2011; 34(6):1262-1276.
[5] Casazza A, Di Conza G, Wenes M, et al. Tumor stroma: a complexity dictated by the hypoxic tumor microenvironment. Oncogene. 2014; 33(14):1743-1754.