1518
Investigating the effect of macromolecular cross-linking and increasing fiber density on the diffusion and viscoelastic properties of extracellular matrix materials using multiparametric MRI1Institute of Cancer Research, London, United Kingdom, 2National Physical Laboratory, London, United Kingdom
Synopsis
Synthetic polymer polyvinylpyrrolidone and fibrous protein collagen were used to investigate the effect of macromolecular cross-linking and increasing fiber density on the physicochemical properties of extracellular matrix models using clinical MRI parameters and torsional rheometry. T1 and T2 decreased with increasing viscoelastic moduli of both materials. Covalent cross-linking of macromolecules by irradiation affected stiffness, but had a smaller effect than polymer concentration on T1, T2 and ADC. Collagen at increasing concentrations sufficient to substantially affect tissue stiffness (reflecting increasing fiber density) affected the structure of water within tissue, (changes in T1 and T2), but did not hinder water diffusion.
Introduction
The tumor extracellular matrix (ECM) differs substantially in its microstructure from normal tissues. Well-established features of the tumor ECM include changing microstructure and density of macromolecules present, including collagen fibers1,2. On imaging, many tumors are characterized by a change in MR relaxation properties in comparison to normal tissues, greater restriction to water diffusion as measured by the apparent diffusion coefficient3 (ADC) and increased stiffness. In fact, ADC has been exploited as a biomarker of tumor grade and aggressiveness in a range of tumor types. However, the impact of changing ECM physicochemical properties on quantitative MR parameters are not clearly understood.
The aim of this study therefore was to investigate the effect of macromolecular cross-linking and increased fiber density on MR parameters and on stiffness of ECM models. We used polyvinyl pyrrolidone (PVP) and collagen I gels as ECM mimetic materials.
Methods
Samples of 2ml volume were prepared and imaged using clinical measurement parameters in 12 well cell culture plates.
PVP in solution has been used extensively as a phantom in MRI4. Therefore, PVP (Mw 1.3x106 Da) solutions were used for cross-linking experiments; solutions of concentrations 15-30w/w% were photo-cross-linked, 7cm from a UV source (254 nm) for 4 hours5.
Collagen I, the most abundant structural protein in the ECM, is used as a 3D cell culture platform and its presence in tumor matrix is well characterized. It was used in increasing concentration (1.6-16 mg/ml) for investigating the effect of decreasing inter-fibre distance. Collagen polymerisation was initiated by increase in pH to physiological pH as previously described6.
MRI data was obtained using a clinical scanner at 1.5T (Siemens, Magnetom Aera) using a hand coil array; HASTE and Resolve EPI DWI (b=0,800), TSE multi-echo (TE=13-419 ms, TR=4000ms), inversion recovery (TE=1.24ms, TI=0-9000 ms). Control samples of water and a 25 w/w% solution of PVP (Mw 55,000 Da) were also imaged, taking one slice that encompassed the entire sample, temperatures were recorded. Images were analysed off-line using in-house software (ADEPT, working under IDL7).
Elastic (G′) and viscous (G″) moduli of samples were measured using a torsional rheometer (AR-G2, TA Instruments) with 8mm parallel plates. This rheometric technique is an established method to measure the viscoelastic properties of materials and provides a standard measurement of materials’ biomechanical properties. Oscillitary frequency sweep (0.1-10Hz) experiments were performed on samples at a controlled strain, previously determined to be within the linear viscoelastic region of the samples. Temperature was matched to MRI.
Results
Effects of cross-linking on biophysical properties: After UV-exposure, photo-cross-linking of PVP is demonstrated by the increase in viscoelastic moduli, G′ is often shown as measure of elastic behaviour of ECM materials. Synthetic PVP solution caused a linear decrease in T1, T2 and ADC with increasing polymer concentration (15-30%, Figure 1) as expected from previous studies of PVP solutions in MRI4. There was a concomitant increase in G′. After UV-exposure, only a slight further decrease in MR parameters was seen compared to non-irradiated samples of the same concentration.
Effects of increased collagen fiber density on biophysical properties: With increasing collagen concentration (1.6mg/ml to 16 mg/ml), there was a 17% decrease in T1, and a 71% decrease in T2 albeit with no evidence of a trend in ADC (Figure 2). G′ increased (230-2190 Pa) as collagen concentration increased. The reduced ADC observed within tumors therefore is unlikely to be due to collagen I if present at concentrations up to 16 mg/ml, although T1 and T2 may change.
Relationship between ADC and stiffness with macromolecular structure: At concentrations resulting in a G’ of ~700 Pa (20% for PVP and 0.4% (4 mg/ml) for collagen, ADC decreased by 40% and 5% respectively, but T2 decreased by 53% and 76% respectively.
Discussion and Conclusion
Synthetic polymer materials have controllable biophysical properties, making them appealing materials to model the matrix. However, they often lack the biological motifs necessary for cellular attachment and proliferation and do not fully replicate the structure of folded proteins.
Covalent cross-linking of macromolecules has a less important effect on T1, T2 and ADC than polymer concentration, although it does affect the elastic properties of materials.
Collagen at concentrations sufficient to substantially affect tissue stiffness affects the structure of water within tissue, as shown by changes in T1 and T2, but does not hinder water diffusion, using clinical DWI sequences.
There is variability in the relationship between MR parameters and viscoelastic moduli for different macromolecules. This could have implications for both the choice of phantom materials and the understanding of the effects of physiological changes in tissue and will be further investigated.
Acknowledgements
CRUK and EPSRC support to the Cancer Imaging Centre at ICR and RMH in association with MRC and Department of Health C1060/A10334, C1060/A16464 and NHS funding to the NIHR Biomedical Research Centre and the Clinical Research Facility in ImagingReferences
1. Nadiarnykh, O., LaComb, R. B., Brewer, M. A. & Campagnola, P. J. Alterations of the extracellular matrix in ovarian cancer studied by Second Harmonic Generation imaging microscopy. BMC Cancer, 2012:10, 94.
2. Lu, P., Weaver, V. M. & Werb, Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol., 2012:196, 395–406.
3. Charles-Edwards, E. M. & deSouza, N. M. Diffusion-weighted magnetic resonance imaging and its application to cancer. Cancer Imaging, 2006:6, 135–143.
4. Jerome, N. P., Papoutsaki, M. V., Orton, M. J., Parkes, H. G., Winfield, J., M., Boss, M. A., Development of a temperature-controlled phantom for magnetic resonance quality assurance of diffusion, dynamic, and relaxometry measurements, Medical Physics, 2016:43, 2998-3007.
5. Lopérgolo, L. C., Lugão, A. B. & Catalani, L. H. Direct UV photocrosslinking of poly(N-vinyl-2-pyrrolidone) (PVP) to produce hydrogels, Polymer, 2002:44, 6217–6222.
6. Nyga, A., Loizidou, M., Emberton, M. & Cheema, U. A novel tissue engineered three-dimensional in vitro colorectal cancer model. Acta Biomater. 2013:9, 7917–7926 (2013).
7. Doran, S. J. et al. Informatics in Radiology Development of a Research PACS for Analysis of Functional Imaging Data in Clinical Research and Clinical Trials. Radiographic's 2012:32, 2135–2150.
Figures
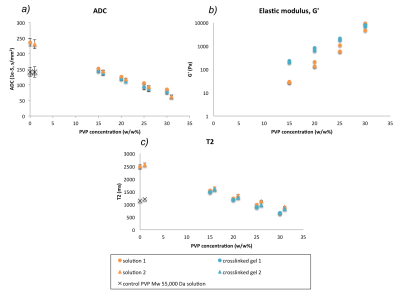
Figure 1: ADC, G′ and T2 of water in PVP cross-linked gels compared to solutions of the same concentration (15, 20, 25, 30 w/w%). G′ measured at 1 Hz. Circles and triangles refer to imaging of samples performed on different days. Error bars on (a) and (c) refer to standard deviation of voxels within the ROI.
As PVP concentration increases, G′ increases (b) and ADC decreases (a), indicating that as PVP becomes more stiff, and diffusion is more hindered. Cross-linking caused a concentration dependent increase in G′ and a small but systematic decrease in both ADC (a) and T2 (c).
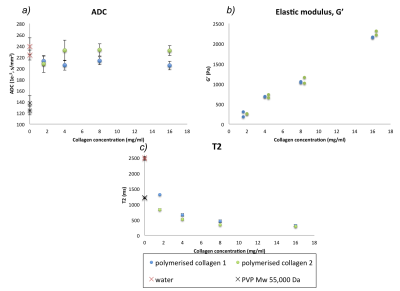
Figure 2: G′, ADC and T2 of water in collagen gels of increasing concentration (1.6, 4, 8, 16 mg/ml). G′ measured at 1 Hz. Blue and green refer to experiments performed on different days. Error bars in (a) and (c) refer to standard deviation of voxels within the ROI.
With increasing collagen concentration, G′ increases (b) and T2 decreases (c), but there is limited change in ADC (a).