1513
Exploring the use of MR Elastography to probe immune cell-stromal interaction in tumour microenvironment1Department of Radiological Imaging, King's College London, London, United Kingdom, 2School of Cancer and Pharmaceutical Sciences, King’s College London, London, United Kingdom, 3School of Cancer and Pharmaceutical Sciences King's College London, London, United Kingdom
Synopsis
There is great, unmet need in understanding and monitoring non-invasively the immune cell changes within the tumour stromal microenvironment during cancer treatment. However there is as yet no reliable non-invasive method of identifying at very early time points patients who are most likely to benefit from this relatively expensive class of treatments which generally are only associated with a clinical response in 25-30% of patients1. We show here in a mouse model that changes 11 days after implantation in the liquid-to-solid ratio (phase angle y) of the tumour biomechanics are indicative for successful immune cell – stromal cell interactions.
Introduction
Recent results demonstrate an enrichment of a type of immune cells known as lymphoid tissue inducer cells (LTi, a subset of innate lymphoid cell class 3, ILC-3) in aggressive human breast cancer subtypes2,3. These immune cells are recruited into the tumour microenvironment (through a chemokine (CCL21)-dependent mechanism), interact with and activate stromal cells to provide signals (involving another chemokine CXCL13) that promote tumour cell motility and invasion into lymphatics and metastasis to lymph nodes. This is one of the first specific examples of how immune cells can interact with stromal fibroblasts (and/or precursors) to enhance tumour cell metastasis. This recent discovery of immune cell-stromal interactions in primary breast tumour3 led us to hypothesize that, by monitoring via MR-Elastography (MRE) the biomechanical properties of the stromal fibroblast activity as a surrogate marker, we may be able to probe immune cell changes in the tumour microenvironment, and thereby quantify at an early time point effects of the therapy. Here, we actually challenge a dogma: the total number of immune cells arriving at the tumour location is small and should in principle not lead to a detectable level of collagen deposition measurably impacting biomechanics at the mesoscopic level. Our preliminary in-vivo data show the contrary giving rise to the hypothesis that immune cells stimulate a vast amount of fibroblasts during their journey throughout the tumour stroma at a migration speed as elevated as 4mm/day.Material & Methods
Previous4 and recent5 in-vivo breast cancer studies demonstrated the use of MRE parameters such as elasticity modulus Gd and viscous modulus Gl for distinguishing benign from malignant breast lesions. Specifically, elevated values of the phase angle correlated with elevated levels of aggressiveness. Here we study tumour biomechanics in an in-vivo mouse model (4T1.2) 11 days after implantation. Control tumours are compared to animals treated with anti-CCL21 blocking antibodies, which prevent immune cells to arrive at the tumour location. 3D MRE is performed on a clinical 3T MRI scanner (Philips Medical Systems) using a microscopy surface coil providing 1mm3 isotropic image resolution at a mechanical excitation frequency of 140Hz.Results
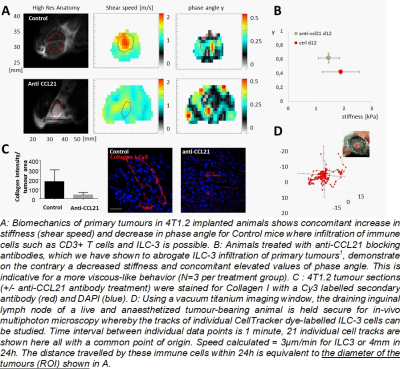
Biomechanics differs strikingly depending on the presence/absence of ILC-3 immune cells (A). Particularly, histopathology showed that normal tumour progression (control) was accompanied with elevated Collagen I deposition (C) and this was diminished in anti-CCL21 treated mice where immune cell arrival was suppressed. The presence of Collagen I shifted tumour biomechanics to elevated stiffness values (non-significant) and lower phase angles (highly significant) indicative for adding spring-like struts in the form of Collagen I to an otherwise viscoelastic material (B). The ILC-3 numbers we have observed in tumour sections are only of 0-56 cells/mm2. In order for such ILC-stromal cell interactions to have a detectable impact on stromal stiffening that is observable by MRE, there would need to be an “amplification” of the signal due to the motility of ILC-3 cells, making extensive contacts with stromal cells, within the tumour microenvironment of breast cancers. We have measured travel distances of up to 4mm within 24h (D) demonstrating that ILC-3 cells can indeed make extensive contacts with stromal cells, within the tumour microenvironment of breast cancers.Conclusions
We published recently that non-small cell lung cancer (NSCLC) xenograft expressing the constitutively active mutant form of EGFR (L858R) demonstrated more fibrogenic potential in the stroma via collagen deposition than NSCLC expressing wild type EGFR6. This was demonstrated using the fibroblast activation marker (alpha- Smooth Muscle Actin (SMA) antibody). Similarly, Isacke et al. have demonstrated changes in tumour-associated fibroblasts in the 4T1 and related syngeneic mouse model, using the pan-fibroblast marker endosialin7. Collectively taken, there is ample evidence that the interaction between immune cells and tumour-associated fibroblasts i) is non-local in space due to the high motility of ILC-3 cells, ii) is leading to Collagen I deposition, iii) can be detected macroscopically via changes in biomechanical properties, and iv) leads to a more solid-like biomechanical appearance.Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 668039.References
[1] Merelli, B., Massi, D., Cattaneo, L. & Mandala, M. Targeting the PD1/PD-L1 axis in melanoma: biological rationale, clinical challenges and opportunities. Critical reviews in oncology/hematology 89, 140-165, doi:10.1016/j.critrevonc.2013.08.002 (2014).
[2] Fu, Z. et al. The crosstalk: Tumor-infiltrating lymphocytes rich in regulatory T cells suppressed cancer-associated fibroblasts. Acta oncologica 52, 1760-1770, doi:10.3109/0284186X.2012.760847 (2013).
[3] Irshad, S. et al. RORgammat+ innate lymphoid cells promote lymph node metastasis of breast cancers. Cancer research, DOI 10.1158/0008-5472.CAN-1116-0598, doi:10.1158/0008-5472.CAN-16-0598 (2017).
[4] Sinkus, R. et al. MR elastography of breast lesions: understanding the solid/liquid duality can improve the specificity of contrast-enhanced MR mammography. Magnetic resonance in medicine 58, 1135-1144, doi:10.1002/mrm.21404 (2007).
[5] Balleyguier, C. & Sinkus, R. Value of whole breast MR Elastography added to MRI for lesion characterization. NMR in biomedicine accepted (2017).
[6] Ortiz-Zapater, E. et al. MET-EGFR dimerization in lung adenocarcinoma is dependent on EGFR mtations and altered by MET kinase inhibition. PloS one 12, e0170798, doi:10.1371/journal.pone.0170798 (2017).
[7] Locard-Paulet, M. et al. Phosphoproteomic analysis of interacting tumor and endothelial cells identifies regulatory mechanisms of transendothelial migration. Science signaling 9, ra15, doi:10.1126/scisignal.aac5820 (2016).