1512
MRI-compatible intravital imaging window for longitudinal imaging of orthotopic mouse ovarian and pancreatic tumor stromaFilip Bochner1, Vishnu Mohan1, Inbal Biton2, and Michal Neeman1
1Biological Regulation, Weizmann Institute of Science, Rehovot, Israel, 2Veterinary Resources, Weizmann Institute of Science, Rehovot, Israel
Synopsis
Longitudinal multi-modal imaging of abdominal organs remains a challenge. In cancer research, where data acquired at multiple spatial and temporal scales is especially valuable, combination of powerful microscopic methods with MRI can yield complementary information about ECM and vascular components of the tumor stroma, both constituting a hallmark of pancreatic and ovarian tumors. Here we present the MRI compatible optical imaging window for longitudinal imaging of ovary and pancreas.
Introduction
MRI and microscopy are complementary tools for preclinical research. MRI enables noninvasive longitudinal imaging of organs and tissues. It provides insight into the tissue structure, metabolism and blood supply both in physiological conditions and pathology. Microscopy performed in live animals enables imaging of cell migration, as well as extracellular matrix remodeling in high resolution. Observing such changes is a key to understand the processes leading to tumor growth and metastasis. Due to development of intravital imaging windows, intravital microscopy can now be also performed repeatedly for prolonged periods of time. Utilization of modern plastic materials enables manufacturing of imaging windows compatible with MRI scanners. Here we present an MRI-compatible imaging window, for longitudinal imaging of mouse ovary and pancreas.Methods
To assure compatibility with MRI we constructed the imaging window using polyetheretherketone (PEEK). PEEK is a bio-compatible, high-strength thermoplastic material with very good mechanical and chemical properties. The imaging window is surgically implanted into the dorso-lateral side of mouse as described [1]. It is stitched to the edges of circular incision made in the skin. The ovary or the pancreas are exteriorized through a small incision in the peritoneum and glued to the window. The coverslip is fixed on top of the organ with a c-ring. Mouse ovarian ID8, eGFP-expressing tumors were grafted to the ovarian fat pad of syngeneic C57Bl mice, 1 day after mounting the imaging window. At days 4 and 6 multiparametric imaging was conducted with MRI (9.4T, Bruker), two-photon (LSM 880, Zeiss) and stereomicroscope using fluorescent and LSI (Laser Speckles Imaging) mode. Fluorescence of ID8 tumors helped to localize the tumors within the ovarian fat pad on T2-weighted images. We were able to quantify the collagen within the tumors using Magnetization Transfer Imaging. Magnetization Transfer Ratio maps were calculated at day 4 and 6 post-implantation. Within the same ROIs, and on the same time points, we respectively quantified the collagen with Second Harmonic Generation imaging by two-photon microscopy. Alternatively, we injected human ES2 ovarian cancer cells into the ovarian fat pad of CD1/nude mice and then followed the collagen deposition within the abdomen, without the imaging window. We marked the ROIs and calculated the MTR at days 6, 9, 12 and 16 after tumor implantation in two abdominal compartments: the surgical metastasis site in the skin and in the ovarian fat pad. For the pancreatic tumor imaging, we injected pancreatic ductal adenocarcinoma cell line (KPC) into the pancreas of C57b mouse with fluorescent blood vessels (VECAD/tdTomato) 1 day after mounting the imaging windo. At days 2, 4, 6 and 8 we conducted two-photon and stereomicroscope imaging using fluorescent and LSI modes as well as with the two-photon microscope.Results and discussion
Longitudinal, multiparametric imaging of the ID8 tumors facilitated with the imaging window yielded information about the tumor vasculature and the ECM. Optical access to the tumor microenvironment enabled us to mark ROIs for calculation of MTR maps with a greater accuracy that in case of regular abdominal imaging. We could observe increased deposition of the collagen within the ovarian tumors over time on the MTR maps, which was confirmed with two-photon microscopy, where formation of new collagen bundles was observed around the invading tumor cells (Fig. 1). Abdominal imaging of fibrosis induced by ES2 tumor cells demonstrated different extents of collagen deposition in the skin surgical metastases compartment and inside the fat pad, where less fibrosis was detected in comparison to the skin (Fig. 2). Intravital, optical imaging of the pancreas yielded similar information about the blood vessels; formation of functional blood vessels over time was directly imaged with stereomicroscope and in addition (Fig. 3), we quantified the morphology of the blood vessels on two-photon z-stacks.Acknowledgements
No acknowledgement found.References
1. Bochner, F., et al., A novel intravital imaging window for longitudinal microscopy of the mouse ovary. Scientific reports, 2015. 5.Figures
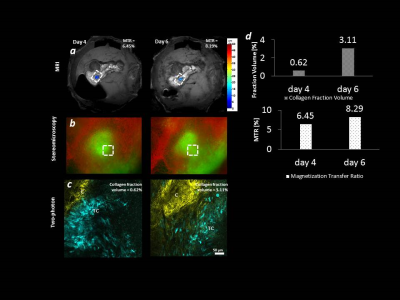
Figure 1: Multimodal
imaging of collagen remodelling in ID8 tumors.
(a) Magnetization transfer ratio maps
of the tumor growing within the ovarian fat-pad (b) Distribution of tumor cells and surrounding blood vessels. (c) Two-photon imaging of collagen
remodelling in the tumor. (d) Quantification of the collagen accumulation in MRI and two-photon
images. Yellow
– collagen (SHG), green (b) or cyan (c) –
tumor cells (EGFP), red – blood vessels (laser
speckles).
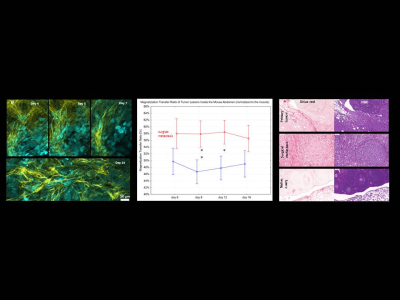
Figure 2 |
Tumor-mediated remodeling of collagen and vasculature (ES2 tumor cells in nude
mice). (a)
Longitudinal imaging of collagen fibers throughout tumor development in the
ovary (ES2 clear cell carcinoma in CD-1 nude mice). (b) MTR
[%] values corresponding to different collagen amounts in several compartments
of mouse abdomen. (c) Sirius Red and H&E staining
of relevant abdominal compartments imaged with MRI. Yellow
– collagen,
cyan – tumor
cells.
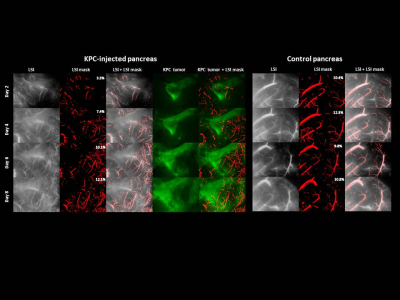
Figure
3 | Longitudinal imaging of KPC tumors with steremicroscope. LSI – Laser
Speckles Imaging depicts blood blow within the growing
blood vessels, LSI mask – binary mask used to quantify the %area of the blood
vessels, KPC tumor – pancreatic ductal adenocarcinoma (PDAC) cells expressing
eGFP.