1509
Using MRI to assess sonic hedgehog pathway inhibition in a genetically-engineered mouse model of adamantinomatous craniopharyngioma1Division of Radiotherapy and Imaging, The Institute of Cancer Research, London, United Kingdom, 2Developmental Biology and Cancer Research Programme, Birth Defects Research Centre, Great Ormond Street Institute of Child Health, University College London, London, United Kingdom, 3Division of Clinical Studies, The Institute of Cancer Research, London, United Kingdom
Synopsis
Expression of sonic hedgehog (SHH) pathway components is enriched in adamantinomatous craniopharyngiomas (ACPs) arising in Hesx1Cre/+;Ctnnb1lox(ex3)/+ mice compared to control pituitaries. An MRI-embedded trial of smoothened inhibitor vismodegib in this genetically-engineered mouse model was undertaken to assess SHH pathway inhibition in ACP. Longitudinal MRI identified accelerated solid tumour growth in response to 28 days vismodegib treatment, which was associated with increased tumour cell proliferation, and resulted in shorter survival. 7 days of treatment induced early tumoural lesions in Hesx1Cre/+;Ctnnb1lox(ex3)/+ pituitaries, resulted in a more undifferentiated and proliferative phenotype, and was associated with an elevated number of cells with clonogenic potential.
Introduction
Adamantinomatous craniopharyngiomas (ACPs) are histologically benign but clinically aggressive pituitary tumours mostly affecting children. ACP, a mixed solid/cystic tumour, is treated with surgery, radiotherapy and cystic drainage, but no specific chemotherapeutics currently exist. Hesx1Cre/+;Ctnnb1lox(ex3)/+ mice, in which oncogenic β-catenin is expressed in the developing pituitary, develop tumours resembling human ACP;1 the radiology and progression of which have been characterised using anatomical and functional MRI.2
Genes involved in the sonic hedgehog (SHH) pathway are enriched in human ACP tissue relative to control tissue; the β-catenin-accumulating cell clusters express SHH, which acts in a paracrine manner to activate the pathway in surrounding tumour cells.3 Both Shh and Gli1 are enriched in the pituitaries of Hesx1Cre/+;Ctnnb1lox(ex3)/+ mice compared to controls; although absolute expression reduces with the time, the differential between control and Hesx1Cre/+;Ctnnb1lox(ex3)/+ mice remains substantial, and significant, at 2 months.
Smoothened (SMO) inhibitor vismodegib (GDC449) is licenced for the treatment of basal cell carcinoma and clinical trials are underway in a number of malignancies, including paediatric medulloblastoma.
We performed an MRI-embedded trial to test the hypothesis that inhibition of the SHH pathway using vismodegib would improve survival of Hesx1Cre/+;Ctnnb1lox(ex3)/+ mice.
Methods
Hesx1Cre/+;Ctnnb1lox(ex3)/+ mice were treated orally with either 100mg/kg vismodegib (n=12) or vehicle (n=11) twice daily for 28 days from ~36 days old (range 31-42 days). Multi-slice T2-weighted images were acquired using a 7T Bruker microimaging system with a 3cm birdcage coil over a 2.5cm field-of-view (RARE; TR=4500ms, TEeff=36ms). MRI was performed on the final day of treatment and at least every 2 weeks thereafter, until mice presented with neurological symptoms or lost condition.
Additional age matched Hesx1Cre/+;Ctnnb1lox(ex3)/+ mice were treated with 100mg/kg vismodegib or vehicle (n=3/group) for 7 days before the pituitary was removed for assessment of the acute effects of treatment.
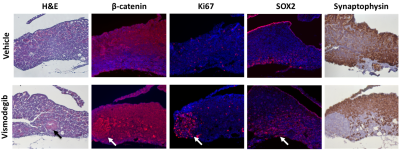
Formalin-fixed, paraffin-embedded sections of pituitary and tumour tissue were stained with haematoxylin and eosin (H&E), or probed immunohistochemically for Ki67 (proliferation), β-catenin, synaptophysin (neuroendocrine differentiation), SOX2 (stem cells) or phospho-histone H3 expression (mitosis).
Results
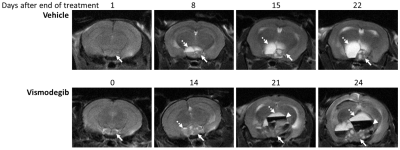
T2-weighted MRI demonstrated enlargement and heterogeneity within the pituitary in Hesx1Cre/+;Ctnnb1lox(ex3)/+ mice in both treatment groups prior to the development of hyperintense cysts, enlargement of a solid portion of the tumour and presentation of hypointense haemorrhagic regions (Figure 1). Substantial intra- and inter-tumoural heterogeneity in T2-weighted signal intensity was observed in both cohorts and no differences in the tumour phenotype were evident between the groups.
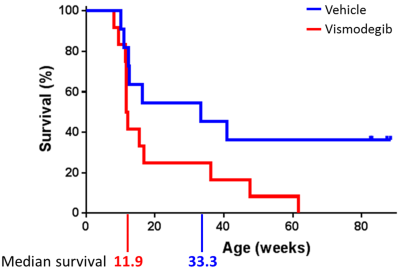
Median survival of animals treated with 100mg/kg vismodegib was significantly shorter than vehicle-treated control mice (11.9 weeks vs. 33.3 weeks; p=0.049, log rank test; Figure 2).
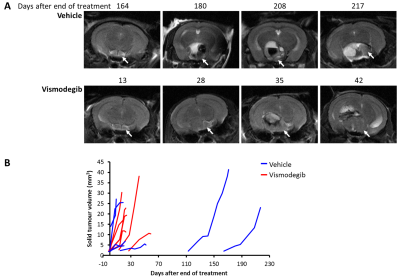
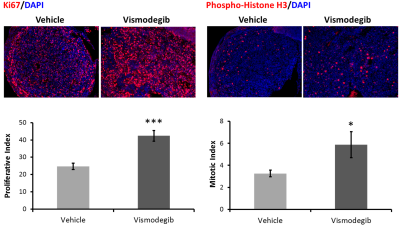
Total lesion and solid tumour volume was calculated from contiguous MR images and demonstrated that the doubling time of the solid component of the tumours was significantly shorter in vismodegib-treated tumours than controls (vismodegib 8.1±1days, vehicle 15.3±3days, p=0.044, unpaired Students t-test; Figures 1 and 3). Consistent with this, proliferation and mitotic indices, as assessed by Ki67 and phospho-histone H3 staining, respectively, were both significantly higher in tumour tissue from vismodegib-treated mice than control mice (n=9/group; Figure 4).
H&E staining of the pituitaries of mice treated with vismodegib for 7 days demonstrated early tumoural lesions that had not been observed previously and were absent from vehicle-treated pituitaries. Immunostaining revealed more Ki67 positive cells in vismodegib-treated pituitaries, which co-localised with β-catenin expression. Fewer synaptophysin positive cells and higher SOX2 expression in vismodegib-treated pituitaries were indicative of undifferentiated lesions with higher stem cell potential, and suggest an increase in putative tumour-initiating cells (Figure 5). In agreement, the pituitaries of vismodegib-treated mice showed higher clonogenic potential in vitro than vehicle-treated controls.
Discussion
MRI investigations of genetically-engineered mouse models represent a powerful strategy to accurately assess tumour response and accelerate preclinical drug development.
Presentation of heterogeneous pituitary tumours with associated cysts and haemorrhage in both treatment groups was consistent with previous imaging studies of Hesx1Cre/+;Ctnnb1lox(ex3)/+ mice.2 Chronic inhibition of the SHH pathway using SMO inhibitor vismodegib in Hesx1Cre/+;Ctnnb1lox(ex3)/+ mice resulted in reduced survival and accelerated solid tumour growth associated with increased proliferation. Short-term treatment induced early tumoural lesions, a more undifferentiated phenotype, and an elevated number of cells with clonogenic potential.
These data are consistent with studies in the KPC model of pancreatic ductal adenocarcinoma, in which knockdown of Shh or chronic treatment with smoothened inhibitor IPI-926 resulted in undifferentiated proliferative tumours and reduced survival.4
Conclusion
This MRI-embedded preclinical trial demonstrates that inhibition of the SHH pathway in Hesx1Cre/+;Ctnnb1lox(ex3)/+ mice increases tumourigenesis and reduces survival by modulating the proliferative capability of tumour-initiating cells and their progeny. Caution should be taken when considering such agents for use in ACP patients.Acknowledgements
We acknowledge the CR-UK support to the Cancer Imaging Centre at The Institute of Cancer Research and The Royal Marsden Hospital in association with the MRC and Department of Health (England) (C1060/A16464), CR-UK Fellowship Grant, the MRC (MR/M125/1) and Children with Cancer UK (15/190).References
1. Gaston-Massuet C, Andoniadou CL, Signore M, et al. Increased Wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proc Natl Acad Sci U S A. 2011;108:11482-11487.
2. Boult JKR, Apps JR, Hölsken A, et al. Preclinical transgenic and patient-derived xenograft models recapitulate the radiological features of human adamantinomatous craniopharyngioma. Brain Pathol. 2017: In press. doi:10.1111/bpa.12525.
3. Andoniadou CL, Gaston-Massuet C, Reddy R, et al. Identification of novel pathways involved in the pathogenesis of human adamantinomatous craniopharyngioma. Acta Neuropathol. 2012;124:259-71.
4. Rhim AD, Oberstein PE, Thomas DH, et al. Stromal Elements Act to Restrain, Rather Than Support, Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2014;25:735-747
Figures