1504
MR-HIFU setup for preclinical treatment of a mouse model of pancreatic ductal adenocarcinoma1Radiology, University of Washington, Seattle, WA, United States, 2Clinical Science MR Therapy, Philips, Andover, MA, United States, 3Applied Physics Laboratory, University of Washington, Seattle, WA, United States, 4Gastroenterology, University of Washington, Seattle, WA, United States
Synopsis
Preclinical studies using animal disease models on clinical MR-HIFU systems are important for human clinical translations but are often very challenging. We developed and tested a set of hardware components to treat a transgenic mouse model of pancreatic ductal adenocarcinoma on our clinical MR-HIFU system. The hardware components include an optimized RF coil, filter, RF switches and coil/animal holder. A gel phantom and a fixed mouse body were sonicated using the developed devices and a mild hyperthermia protocol on a 3T MR-HIFU system. Pulse sequences for multi-parametric MRI were also tested to acquire optimum signal-to-noise ratio on the samples.
Introduction
Pancreatic ductal adenocarcinoma (PDA) is the fourth leading cause of cancer related death with a 5-year survival rate of 8% [1]. MR-HIFU has recently demonstrated potential treatment efficacy of some diseases including PDA [2]. Both mild hyperthermia generated by HIFU and pulsed HIFU could help provide effective treatment options for the deadly disease. To test the efficacy of a HIFU treatment for PDA, we utilized a genetically engineered KPC mouse model of PDA. However, one of the challenges in performing the test is developing an appropriate experimental setup using the mouse model on a clinical MR-HIFU system.
We used a 3 Tesla (T) clinical MR-HIFU system for the treatment of the KPC mouse model of PDA with the goal of eventual clinical translation. Research MRI scanners at magnetic fields higher than 3T equipped with HIFU options would provide much quicker applications of MR-HIFU methodology to mouse studies but the findings obtained at higher fields may not be directly translatable for a human clinical trial on a human clinical scanner. Performing studies using a 3T clinical MR-HIFU system allows for possible direct clinical translation. In this study, we designed and fabricated an optimized RF coil, filter, RF switches and coil/animal holder for HIFU treatment on transgenic mouse model of pancreatic ductal adenocarcinoma (PDA).
Methods
A surface coil was fabricated to image a mouse pancreas in vivo at 128 MHz. The coil surface was insulated and in a waterproof condition for ultrasound gel application during the HIFU procedure. The RF coil accommodates a KPC mouse body and ultrasound (US) gel on top of a coil/mouse holder and a degassed water tank that is positioned above a membrane of the HIFU patient table. Images were acquired on a clinical 3T MRI scanner (Ingenia, Philips, Best, the Netherlands) equipped with a clinical HIFU system (Sonalleve V2, Profound Medical, Mississauga, ON, Canada). We fabricated a high pass filter to minimize potential interaction from HIFU sonication at 1.2 MHz. We also constructed RF switches for the filter to improve signal-to-noise ratio (SNR) and to dampen potential interaction of the filter during MRI acquisitions.Results
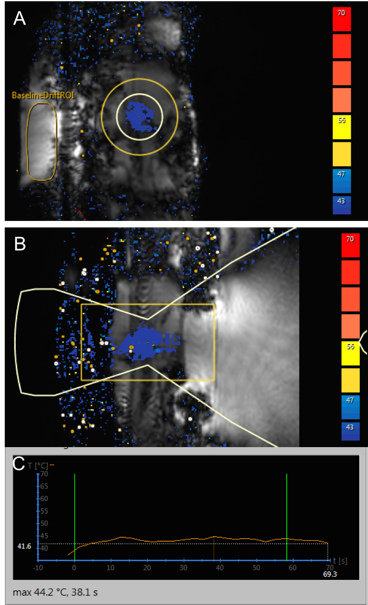
A 1H MR-HIFU mouse body RF receive coil was constructed for high resolution mouse body MRI at 3T to identify tumors (see Figure 1). The coil was tested with a gel phantom on our 3T Ingenia MRI scanner. A degassed water tank was fabricated for MR-HIFU experiments with mice. Plastic membranes were placed on both the top near the RF coil and the bottom of the water container which fits onto the acoustic window of the HIFU table. Figure 2 shows several additional components for the RF coil including a high pass filter, pre-amplifier and RF switches. All these components were enclosed in a shielded box shown in Figure 2. The high pass filter was to minimize potential interaction of US signals at 1.2 MHz to 1H MR images acquired at 128 MHz. We fabricated the RF switches to achieve more complete separation between the RF coil and high pass filter. We discovered some MRI pulse sequences were severely hampered when the high pass filter was functioning. A flip switch was installed on the RF coil connector to remedy this situation. Our RF coil system has two different modes: an MRI mode and an MR-HIFU sonication mode which are controlled by the RF switches. When we conduct multi-parametric MRI, the RF coil is set to the MRI only mode to generate high resolution images. Figure 3 displays a T1-weighted image for both a mouse body and a gel phantom. The T1-weighted image was selected from multi-slice 2D slices showing detailed features of the animal body. Then, during the HIFU sonication mode, mild hyperthermia was successfully generated on the PDA tumor region with the temperature maintained at around 41°C as shown in Figure 4.Discussion
Our results show that the mouse RF coil setup and holder provide high SNR for in vivo MR imaging and stable conditions for MR thermometry during HIFU sonications. The RF switches are useful in performing multi-parametric MRI for pre- and post-HIFU without an adverse effect potentially induced by the high pass filter.Conclusion
We demonstrated our developed experimental setup would provide optimal conditions on a clinical 3T MR-HIFU system for our preclinical study on the genetically engineered mouse model (KPC) of human PDA. We plan to use the setup for future HIFU treatments on KPC mice for targeted drug delivery. Findings obtained from this study have the potential for direct translation to human clinical trials.Acknowledgements
This work was supported by National Institutes of Health R01 CA188654.References
- Siegel R, Miller K, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30.
- Farr N, Wang Y, D'Andrea S, Starr F, Partanen A, Gravelle K, McCune J, Risler L, Whang S, Chang A, Hingorani S, Lee D, Hwang J. Hyperthermia-enhanced targeted drug delivery using Magnetic Resonance-guided focused ultrasound: a pre-clinical study in a genetic model of pancreatic cancer. International Journal of Hyperthermia in press (2017).
Figures