1495
Detecting T1-based signal reduction in focused ultrasound heating of bone at 1.5T using a 3D spiral ultra-short echo time sequence1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA, United States, 3Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, United States, 4Application Development, Siemens Healthcare, Erlangen, Germany
Synopsis
MR-guided Focused Ultrasound (MRgFUS) is used transcranially to ablate brain tissue for the treatment of essential tremor and Parkinson's disease symptoms. Proton resonance frequency shift MR thermometry detects changes in temperature in tissues with sufficiently long T2, but fails to detect heating in the cortical bone of the skull. T1-based MR thermometry uses T1 mapping to observe a linear increase in T1 with temperature but requires long acquisitions. We demonstrate a thermometry method using the linear relationship between signal magnitude from a T1-weighted 3D Spiral Ultra-short Echo Time sequence and temperature in focused ultrasound heated bone with improved temporal resolution.
Introduction
MR-guided Focused Ultrasound (MRgFUS) uses MRI to provide structural information for treatment planning in transcranial FUS procedures. Tissue temperature can also be measured to ensure precise lesion formation using MR thermometry. Typically, MR thermometry makes use of the Proton Resonant Frequency (PRF) shift, where an increase in temperature leads to increased electron shielding, causing a frequency shift from baseline proportional to the temperature of the object. The resulting image phase shift is relatively small and increases with TE 1. PRF-shift thermometry is impractical in tissues with short T2, as the signal decays too quickly to detect the phase change from temperature. In transcranial MRgFUS, PRF-shift thermometry cannot detect heating in the skull, which can lead to pain, burns, and premature cessation of treatment 2. Ultra-short Echo Time (UTE) imaging allows for signal detection of cortical bone despite its short T2, but the ultra-short TE does not allow for PRF-shift thermometry. T1-based thermometry has been used as an alternative mechanism to measure heating in bone 3. Han et al found that T1 increases linearly by a factor of 1% per °C of heating using 3D radial-trajectory variable flip-angle T1 maps 1. Fielden and Miller have shown that signal magnitude decreases with heating in bone 3,4. Here, we hypothesize that the relationship between signal reduction and temperature can be modeled as linear in a FUS heated ex-vivo bovine bone sample using a 3D spiral UTE sequence.Methods
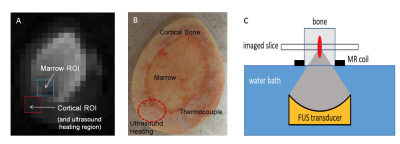
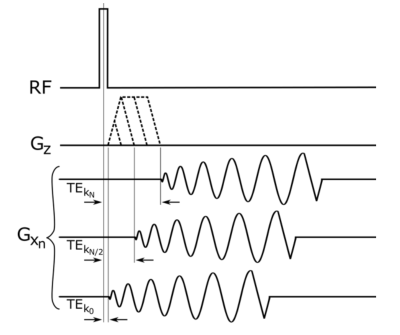
A sample of ex-vivo bovine femur was obtained from a cow butchered the day prior to experimentation. A fiberoptic thermocouple was placed in the cortical bone to measure absolute temperature changes (Fig. 1). The bone was placed on an ultrasound transparent film above a water bath of a RK-100 transducer (FUS Instruments Inc.,Toronto) (Fig. 1c). The system is a small animal focused transducer with a focal spot of approximately 1 mm. A flexible surface receive coil was placed above the sample. Imaging was performed using a prototype 3D spiral UTE pulse sequence (Fig. 2) 4 on a 1.5 T scanner (MAGNETOM Avanto, Siemens Healthcare, Erlangen, Germany). The bone was ablated with a 45W continuous sonication six times for 135 seconds. Imaging began immediately after each ablation. Imaging parameters were: flip angle = 44°, matrix 96x96x16, slice thickness = 3 mm, TR = 11 ms, TE = 50 µs, and 203 linear variable density spiral interleaves of 0.4 ms duration each with sampling density decreasing from 1.0 at the center of k space to 0.7 at the edge. Heating was verified to reach a maximum of 60.2 °C with the internal thermocouple. A 3x3 region of interest around the ablated cortical bone and in the heated marrow was used to measure mean signal intensity for different temperatures (Fig.1b).Results
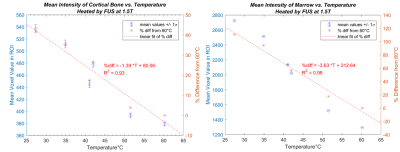
A linear reduction of signal with increasing temperature was observed in both cortical bone and in marrow from 27.4-60.2 °C (Fig. 3). The mean signal difference of cortical bone (relative to 60.2 °C), analogous in composition to a patient’s skull, decreased by 1.39% per °C with a strong correlation (R2 = 0.93) and substantial mean SNR (>20). The mean signal difference of marrow (analogous to marrow or fat in or near the skull) decreased by 3.63% per °C (R2 = 0.98). This linear model supports the hypothesis that signal decrease (T1-weighted) can be approximated as linearly dependent on temperature over the range we investigated.Conclusion
As reflected by the FDA’s recent approval of MRgFUS treatment of essential tremor (2016) and of Parkinson’s disease (2017), the clinical rise of MRgFUS necessitates improvements in the accuracy of MR thermometry to address concerns of unintended dangerous heating 2. 3D spiral UTE allows fast volumetric imaging capable of covering a large FOV within the time between clinical transcranial sonications 4. Our results found a linear relationship between temperature and signal at 1.5T. These preliminary results thus show the possibility of whole-brain T1-based thermometry, which can increase treatment safety by enabling dose monitoring of the skull during treatment. As the increase in T1 with temperature increases with field strength, higher field imaging may provide better temperature contrast 5. Further sequence development to improve the temporal and spatial resolution will continue to improve MR thermometry and thus increase successful outcomes in MRgFUS.Acknowledgements
Steven Allen for editing suggestions, William Garrison for experimental assistanceReferences
[1] Han M. et al. ISMRM 2014;22: p0262.
[2] Cohen-Inbar O. et al. World Neurosurg. 2016;91: p661-665
[3] Miller GW. 3rd Int Symposium on FUS 2012; p65-BN.
[4] Fielden S. et al. ISMRM 2015;23: p3867.
[5] Rieke, V. & Butts Pauly, K. JMRI 2008; 27: p376–390.
Figures