1484
Development of a Tissue Mimicking Phantom for Focal Laser Ablation of the Prostate1Department of Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States, 2Center for Advanced Surgical & Interventional Technology, University of California, Los Angeles, Los Angeles, CA, United States, 3Department of Urology, University of California, Los Angeles, Los Angeles, CA, United States, 4Department of Radiology, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
There is a need to further develop real-time feedback systems for monitoring focal laser ablation (FLA). Here we have developed a tissue mimicking phantom to facilitate research on the use of magnetic resonance thermometry (MRT) and interstitial thermal probes as feedback systems. The tissue mimicking phantom was designed to match the optical and thermal properties of prostatic tissue at 980nm. The thermal response of the phantom to FLA was then compared to previously acquired clinical data and found to be qualitatively and quantitively similar to prostatic tissue. MRT and real-time quantification of damage zone progression are also demonstrated.
INTRODUCTION
Focal laser ablation (FLA) is a minimally invasive procedure which has been used to treat tumors in many organs including prostate1–3, breast4 and liver5. Cancer control can be achieved with minimal damage to surrounding tissue; however, there is a need to develop a feedback system to enable real-time quantification of the coagulation zone. Both magnetic resonance thermometry (MRT)1 and interstitial thermal probes (ITP)6 present promising approaches. To aid in further development of these techniques we have created a tissue-mimicking phantom with similar optical and thermal properties to prostatic tissue.METHODS
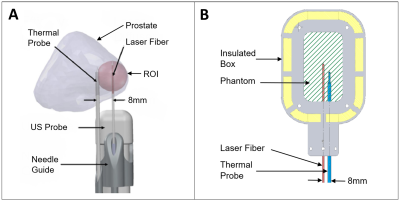
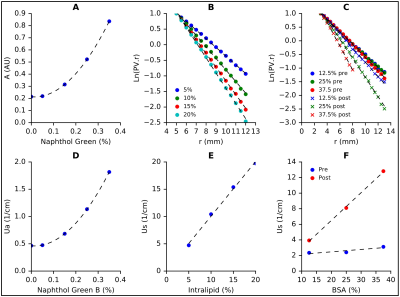
A polyacrylamide gel (PAG) was chosen due to its high melting point, optical transparency, and appropriate thermal properties7. Naphthol Green B (NGB) (Sigma-Aldrich, MO) was used to alter the absorption coefficient (μa) as it readily dissolves in water. The necessary concentration of NGB was determined using a spectrophotometer to measure absorbance (A) in 5 PAGs doped with 0%, 0.05%, 0.15%, 0.25% and 0.35% NGB respectively. To quantify the relationship between Intralipid-20% (Fresenius Kabi, Sweden) and the reduced scattering coefficient (μ's), an optical measurement apparatus (Fig. 1) was constructed. Using custom-built software and a microcontroller, the measured photovoltage (PV) was recorded every 500µm as the dosimetry probe was advanced towards the source probe. The fluence rate for an isotropic point source (Φ) and the effective attenuation coefficient (μeff) are given by equations 1 and 2 respectively (Fig.1-inset). Given that , and is known, can be found from the relationship between ln(PV*r) and r. Bovine serum Albumin (BSA) was used to induce dynamic scatter, or an increase in μ's at elevated temperatures. PAGs doped with NGB and BSA (Boval Co. TX) were tested in the fluence box to determine μ's before and after coagulation.
Finally, the optimized phantom was tested by performing FLA under the same conditions previously utilized in a clinical trial6 (Fig. 2). The coagulation zone was tracked in real-time using a 3T scanner (Prisma, Siemens) with a T2-weighted turbo spin echo (TSE) cine sequence (TE = 15ms, TR = 390, resolution = 0.9*0.9*2mm3, acquisition time = 2s). After laser deactivation, a higher resolution T2-weighted TSE was acquired (TE = 52ms, TR = 2000, resolution = 0.4*0.4*1mm3). The phantom was then sliced in half along the fiber trajectory and photographed. This experiment was repeated in an identical phantom under gradient echo proton-resonance frequency MRT.
RESULTS
At 980nm, the μa and μ's of prostatic tissue are 0.66 cm-1 and 8.1 cm-1 respectively8 with μ's increasing up to three-fold due to ablation9,10. Fig. 3A shows the relationship between NGB concentration and absorbance. This data was used to plot μa vs NGB concentration (Fig. 3D) and by interpolation the required concentration of NGB was found to be 0.14%. The slope of the curves given in Fig. 3B&C is equal to μeff. By using eqn. 2 (Fig.1-inset), μ's was plotted as a function of Intralipid-20% (Fig. 3E) and BSA (Fig. 3F) concentration. To ensure a 100% increase in μ's post hyperthermia and a native μ's of 8.1 cm-1, the desired concentrations of Intralipid-20% and BSA were 5.39% and 32.5% respectively.
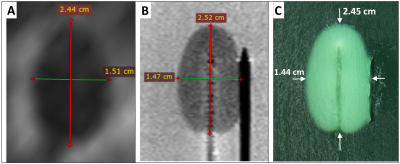
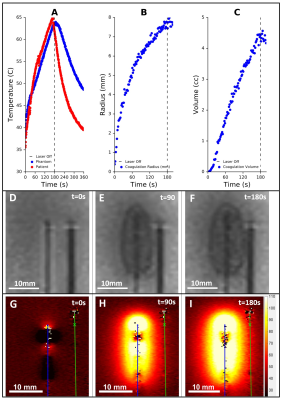
Fig. 4 compares the ablation zone from a patient (A) with the phantom under MRI (B) and direct visualization (C). The shape approximates an ellipse with the maximum variation in major and minor axes found to be 0.8 mm and 0.7mm respectively. Fig. 5A compares the temperature rise, at 8mm from the laser, in a patient and in the phantom. Fig. 5 B&C show the increase in coagulation radius and volume during ablation as calculated from the cine T2 sequences shown in Fig. 5 D-F. Fig. 5 G-I show the increase in temperature as quantified by MRT.
DISCUSSION
The changes in the phantom induced by FLA were both qualitatively and quantitatively similar to prostatic tissue. The dimensions of the phantom’s coagulated zone were nearly identical to the non-perfused region observed in patients. Small variations in thermal response were observed between the patient and phantom, which may be attributed to the uncertainty in ITP placement in the clinical study due to needle deflection. The results, demonstrate the utility of the phantom for real-time tracking of ablation zone growth (Fig. 5B-C) and as medium for analysis of MRT (Fig. 5G-I).CONCLUSION
This study presents a novel tissue mimicking phantom with optical and thermal properties similar to prostatic tissue. The phantom provides a robust platform for the study of techniques such as MRT for monitoring FLA.Acknowledgements
Funding support was provided by NIH/NCI 1R01CA218547References
1. Natarajan, S. et al. Focal Laser Ablation of Prostate Cancer: Phase I Clinical Trial. J. Urol. 196, 1–8 (2015).
2. Lindner, U. et al. Image guided photothermal focal therapy for localized prostate cancer: phase I trial. J. Urol. 182, 1371–1377 (2009).
3. Oto, A. et al. MR Imaging – guided Focal Laser Ablation for Prostate Cancer : Phase 1 Trial. 267, (2013).
4. Haraldsdottir, K. H. et al. Interstitial laser thermotherapy (ILT) of breast cancer. Eur. J. Surg. Oncol. 34, 739–745 (2008).
5. Dick, E. A. et al. MR-guided laser thermal ablation of primary and secondary liver tumours. Clin. Radiol. 58, 112–120 (2003).
6. Natarajan, S. et al. Focal Laser Ablation of Prostate Cancer: Feasibility of Magnetic Resonance Imaging/Ultrasound Fusion for Guidance. J. Urol. 198, 839–847 (2017).
7. Negussie, A. H. et al. Thermochromic tissue-mimicking phantom for optimisation of thermal tumour ablation. Int. J. Hyperthermia 6736, 1–5 (2016).
8. Takada, J., Honda, N., Hazama, H. & Awazu, K. Ex vivo evaluation of safety and efficacy of vaporization of the prostate using a 300 W high-power laser diode with the wavelength of 980 nm. Laser Ther. 23, 165–172 (2014).
9. Jaywant, S. et al. Temperature dependent changes in the optical absorption and scattering spectra of tissues: correlation with ultrastructure S. Jaywant, B. Wilson, M. Patterson, L. Lilge, T. Flotte, J. Woolsey and C. McCulloch. in Laser-Tissue Interaction 1882, 218–229 (1993).
10. Nau, W. H., Roselli, R. J. & Milam, D. F. Measurement of thermal effects on the optical properties of prostate tissue at wavelengths of 1, 064 and 633 nm. Lasers Surg. Med. 24, 38–47 (1999).
Figures